Inicio » Medicine preparation » Biology
Archivo de la categoría: Biology
Enzymes as Catalysts
Metabolic reactions need activation energy to either build or break down molecules. Cells also use special proteins that aid metabolic reactions. These proteins, called enzymes, work by speeding up a chemical reaction. This chemical activity increases the reaction rate, or rate at which a reaction occurs, measured in terms of reactant used or product formed per unit time (while existing conditions remain unchanged). Some of the earliest studies on enzymes were performed in 1835 by Swedish chemist Jon Jakob Berzelius, who termed their chemical action “catalytic.”
Enzymes and the Catalytic Cycle
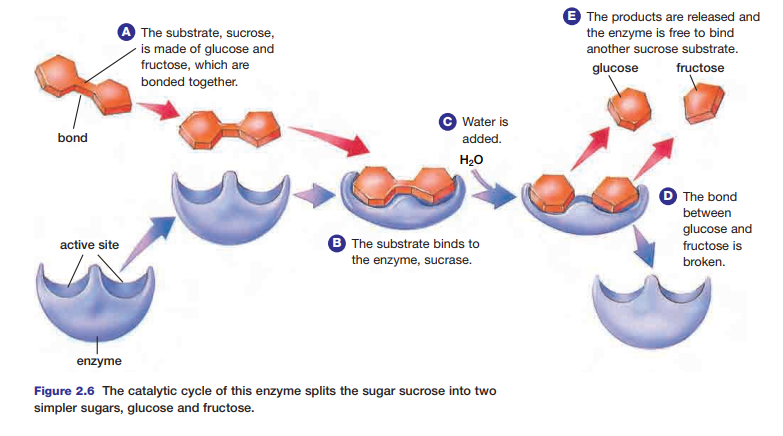
The acceleration of a chemical reaction by some substance, which itself undergoes no permanent chemical change, is called catalysis. The catalysts of metabolic reactions are enzymes, which are involved in almost all chemical reactions in living organisms. Without enzymes, metabolic reactions would proceed much too slowly to maintain normal cellular functions. Consider the hydrolysis of sucrose, an exothermic reaction. A solution of sucrose dissolved in water could sit for years without showing signs of hydrolysis. If the enzyme sucrase is added to the solution, the enzyme speeds up the reaction millions of times, so that all of the sucrose will be hydrolyzed in several seconds.
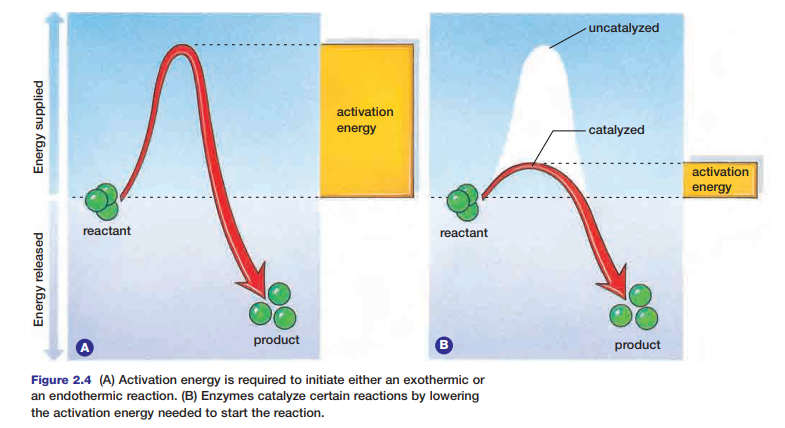
Enzymes speed up reactions by lowering the amount of activation energy needed. Thus, less energy is required for the reaction to begin. The action of an enzyme on an exothermic reaction is illustrated in Figure 2.4.
Cells carry out a large number of different biochemical reactions; many of these reactions require a specific enzyme in order to take place. Different sets of enzymes are responsible for catalyzing different chemical reactions. Oxidative
enzymes (oxidoreductases) work to catalyze oxidation-reduction reactions. Hydrolytic enzymes
(hydrolases) catalyze the addition of water in reactions and split molecules into simpler forms. These simpler molecules may be used to build other molecules or may be excreted from the cell. For example, the lysosomes of cells contain many hydrolytic enzymes. Tasks such as breaking down nucleotides, proteins, lipids, and phospholipids are each carried out by a specific hydrolytic enzyme. Other enzymes remove carbohydrate, sulfate, or phosphate groups from molecules.
Synthesis reactions that build structures such as proteins, nucleic acids, hormones, glycogen, and phospholipids all require the use of enzymes. The enzyme DNA polymerase, for example, is needed for DNA replication, which precedes mitosis. Each chemical reaction in cellular respiration requires a specific enzyme. Deaminases remove the amino
groups from amino acids so the remainder of the molecule can be used as an energy source. Enzymes also help to split long-chain fatty acids into smaller compounds, which are used as an energy source and broken down by the process of cellular respiration.
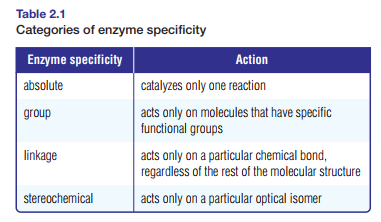
Blood clotting, the formation of angiotensin II to increase blood pressure, and the transport of carbon dioxide in the blood all require specific enzymes. Tables 2.1 and 2.2 show categories of enzyme specificity and modes of action.
A reactant in any given enzymatic reaction is called a substrate for that specific enzyme. Some enzymes catalyze one individual reaction; this is the case with peroxidase, an enzyme that decomposes hydrogen peroxide into water and
oxygen. Reactions within cells, however, are often part of a metabolic pathway (series of linked reactions), beginning with one substrate and ending with a product. Such metabolic pathways can involve many reactions, which often include other pathways. Each step of a metabolic pathway, or each constituent reaction of the pathway, needs its
own specific enzyme.

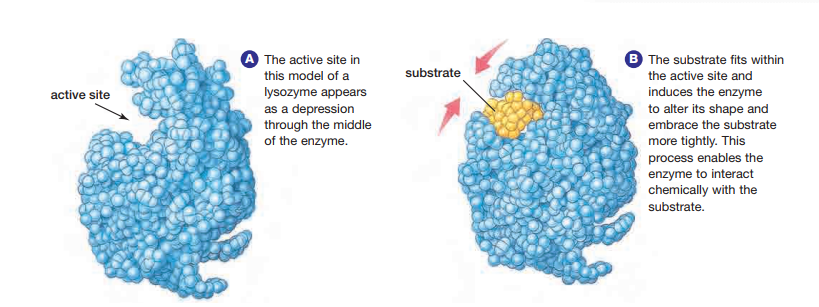
To understand how enzymes work, consider that the key to enzyme function is enzyme structure. Enzymes are globular proteins with depressions on their surfaces, as shown in Figure 2.5. These depressions are called active sites. Active sites are places where substrates fit and where catalysis occurs. Active sites are not static receptacles.
Substrates fit closely into active sites because enzymes can adjust their shapes slightly to accommodate the substrate. This process involves a subtle change in conformation, or three-dimensional shape, of the enzyme when the substrate binds to it. Multiple weak bonds between the enzyme and the substrate are involved in this process. The change in shape of the active site to accommodate the substrate is called induced fit. This process may bring specific amino acid functional groups on the enzyme into the proper orientation with the substrate to catalyze the reaction see Figure 2.5
The combination of the substrate and the enzyme itself forms a compound called an enzyme–substrate complex. Swedish chemist Svanté Arrhenius first hypothesized about the enzyme–substrate complex in 1888, proposing that
there must be a stage during catalysis when the enzyme and the substrate join together. Modern laboratory experiments have confirmed his hypothesis. In many cases, the enzyme–substrate complex is held together by such bonds as hydrogen bonds and weak ionic bonds. The polar and non-polar groups of the active site attract compatible groups on the substrate molecule.
These attractions effectively lock the substrate molecule in the active site. Once in the active site, the substrate is subject to necessary collisions, bond breaks, and bond formations that must take place to form the product molecule. This reaction can be anabolic or catabolic, depending upon the enzyme. Once the product molecule has been
formed, it is released from the enzyme–substrate complex. The enzyme is now able to accept another substrate, and begin the process anew. This cycle is known as the catalytic cycle. Figure 2.6 shows the catalytic cycle involving sucrose and the enzyme sucrase.
There are several methods by which enzymes reduce the activation energy needed to break the bonds in a substrate. In the enzyme–substrate complex, the substrate molecules experience
physical stress. The R-groups in the active site of an enzyme are able to stress the bonds of the substrate. There is bending and stretching of bonds that hold the molecule and the active site together. In this case, the activation energy is lowered because the bonds within the molecule have become weaker, reducing the amount of energy needed to break them.
Another way in which the active site of an enzyme may lower activation energy involves special amino acids that line the active site. These amino acids have reactive R-groups that can aid in the transfer of hydrogen ions to or from the substrate. For example, the active sites of hydrolytic enzymes, such as those within the lysosome, often provide
acidic and/or basic amino acid groups at precisely the correct orientations required for catalysis. The yeast enzyme, intertase (also known as betafructofuranosidase), is a hydrolytic enzyme that speeds up the breakdown of sucrose into the products glucose and fructose. Some other enzymes provide amino acid groups at their active sites that can accept electrons, while others are attracted to atomic nuclei of the substrate.
This process can form a temporary attraction with the substrate. In this state, the substrate is less stable and can more easily react to form the product. Some enzymes may facilitate the correct reaction by bringing two different substrates together in the appropriate orientation to each other.
An oxidative enzyme (such as cytochrome P450s) catalyzes the transfer of electrons from substrates to oxygen molecules. Substrates for these enzymes are often referred to as hydrogen donors because hydrogen ions along with electrons are taken from the substrate. Cytochrome P450s is most common in the endoplasmic reticulum of liver cells. In these cells, the enzyme helps to metabolize toxins, as well as fat-soluble vitamins such as A, D, and E.
Enzyme Activity
As you have learned, enzymes lower the activation energy required to start a chemical reaction. The activity of enzymes, however, can be influenced by environmental factors, such as pH and temperature.
The shape of an enzyme is determined by hydrogen bonds, which hold peptide chains in the enzyme in a specific orientation. As well, all enzymes contain segments that are hydrophobic. The hydrogen bonds in an enzyme and any hydrophobic interactions that parts of the enzyme may experience are easily affected by changes in temperature. Enzyme activity increases as temperature increases, but only up to a maximum point. If the temperature increases beyond a critical point, enzyme activity declines rapidly (see Figure 2.7). When this occurs the enzyme has been denatured. When an enzyme is denatured by excessive heat, its shape changes and it can no longer bind to its substrate.

Most human enzymes function best between 35°C and 40°C. Below this temperature range, enzymes are less flexible and therefore less able to provide an induced fit to substrates. Above this range, the bonds become weaker and less able to hold the peptide chains in the enzyme in the proper orientation. Some bacteria, however, can function at temperatures as high as 70°C. These bacteria live in and around hydrothermal vents, which are fissures in the Earth’s crust on the ocean floor that release hot water and gases. The bacteria are able to survive in these environments because the bonds between peptide chains in their enzymes are relatively strong and able to withstand the extreme temperatures. These enzymes are therefore called thermostable enzymes.
Thermostable enzymes could operate above the growth temperature for pathogens that otherwise can contaminate foods. Potential applications of this knowledge might include development of food products that could be processed at higher temperatures, and are more resistant to microbial contamination (such as E. coli). Thermostable enzymes may also be useful in drug synthesis.
Such enzymes may be able to catalyze reactions more effectively, affording higher productivity. They may also last longer and could possibly be re-used. In the next Thinking Lab, you will conduct research into the sources of enzymes in foods.
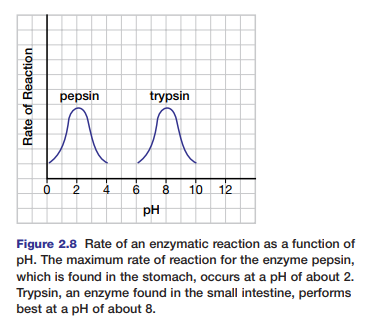
Each enzyme also works optimally (best) at a specific pH. Figure 2.8 shows the activity ranges for the enzymes pepsin and trypsin at different pH levels. At pH values where the enzymes work optimally, the enzymes have their normal
configurations. The bonds that hold peptides in position in the enzyme are sensitive to hydrogen ion concentrations. A change in pH can alter the ionization of these peptides and disrupt normal interactions. Under extreme conditions of pH, the enzyme will eventually denature. Most enzymes function best in the pH range of 6 to 8. Pepsin, which digests proteins in the human stomach, works best under very acidic conditions (pH of 2).
In the next investigation, you will design an experiment to study the effects of temperature and pH on enzyme activity.
Enzyme Inhibitors and Allosteric Regulation
In addition to the environmental factors of pH and temperature, various substances can inhibit the actions of enzymes. Inhibitors are chemicals that bind to specific enzymes. This results in a change in the shape of the enzyme that causes the enzyme to shut down its activity. In cells, enzyme inhibition is usually reversible; that is, the inhibitor is not
permanently bound to the enzyme. Inhibition of enzymes can also be irreversible. For example, hydrogen cyanide, a powerful toxin, is an inhibitor for the essential enzyme cytochrome c oxidase.
Toxins, such as hydrogen cyanide, typically bind (either covalently or non-covalently) so strongly with an enzyme that the enzyme cannot bind with its substrate. Some poisons that result in irreversible enzyme inhibition do not combine with the enzyme; instead, they destroy enzyme activity by chemically modifying critical amino acid R-groups.
Other toxins, such as venom from the Malayan it viper (Calloselasma rhodostoma) (shown in Figure 2.9 on the next page), are enzyme inhibitors that can help people overcome the effects of a stroke. Strokes are caused by blood clots in the brain, which can result in mental and physical debilitation. A substance called ANCROD, derived from this venom, contains enzyme inhibitors that prevent blood clots from forming. In 1999, pharmaceutical researchers found that more than 40% of stroke patients who received ANCROD recovered all of their mental faculties. Other venom, such as scorpion venom, is being used to treat autoimmune disorders.

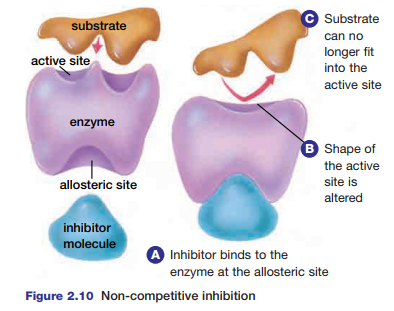
There are two kinds of inhibition that can affect the activity of enzymes. In non-competitive inhibition, an inhibitor molecule binds to the enzyme at a site known as the allosteric site. As a result, the three-dimensional structure of the enzyme is altered, which prevents the substrate from binding to the active site (see Figure 2.10). Most metabolic
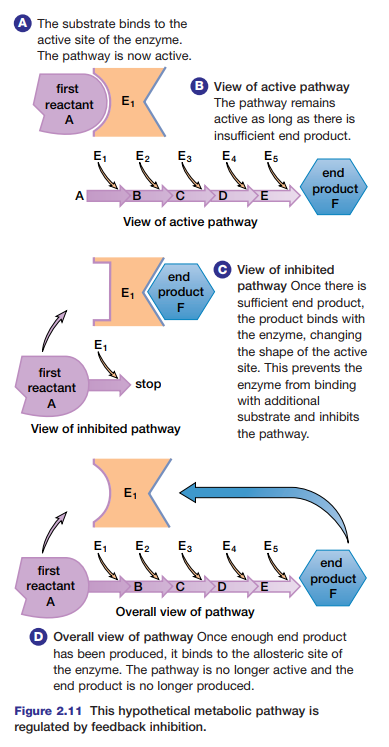
pathways are regulated by feedback inhibition. This is a type of non-competitive inhibition in which the end product of the pathway binds at an allosteric site on the first enzyme of the pathway. In this way, non-competitive inhibitors can play a key role in the normal functioning and regulation of metabolic pathways. Study Figure 2.11 to learn how a metabolic pathway is regulated by feedback inhibition.
Figure 2.10 Non-competitive inhibition
Molecules that promote the action of enzymes can also bind to the allosteric site. These molecules are known as activators. The activity of any enzyme can change, depending on the number of activators and inhibitors in its environment. The regulation of enzyme activity by inhibitors and activators is known as allosteric regulation.
Competitive inhibition involves chemical compounds that bind to the active site of the enzyme and inhibit enzymatic reactions. The compounds compete with the true substrate for access to the active site. This competition is possible because competitive inhibitors are very similar in shape and structure to the enzyme’s substrate. The metabolic pathway can only be restored if the substrate concentration is increased so that the substrate is more likely to enter the active sites than is the inhibitor. Penicillin is a commonly used competitive inhibitor. It works by bonding to the active site of transpeptidase, the enzyme involved in bacterial cell wall construction.
When penicillin transpeptidase inhibits, a bacterial cell cannot divide successfully, and infectionis prevented.
Protease inhibitors are a relatively new class of ompetitive inhibitors that interfere with the normal activity of protease enzymes. Molecular modelling played a major role in the research and design of effective protease inhibitor molecules. Figure 2.12 shows the general appearance and behaviour of protease and protease inhibitors. These inhibitors have been used to dramatically reduce the level of human immunodeficiency viruses (HIVs) in AIDS patients. HIVs infect host cells, such as the T-cells of the human immune system. The virus does this by injecting its genetic material into the host cell. The virus DNA then commandeers the cell’s cellular processes to make polyproteins. The protease HIV enzyme then cuts these polyproteins into smaller structural proteins and enzymes that will be used to make new HIVs. The snipping or cleavage of polyproteins involves a hydrolysis
reaction that uses a water molecule for every bond that is broken in the substrate molecule. HIV protease inhibitors are similar in chemical composition and structure to the HIV polyprotein. The inhibitor molecules bind tightly to the active
site of HIV protease enzymes. This process prevents the enzymes from cutting the actual HIV polyproteins to form new HIVs. The HIV protease enzyme is composed of two identical peptide halves. The enzyme’s active site is located in the
depression formed where the two halves join.
Cofactors and Coenzymes: Non-protein Helpers
The final manner in which enzymes are regulated comes in the form of cofactors. Cofactors are inorganic ions and organic, non-protein molecules that help some enzymes function as catalysts. The inorganic ions are metals such as copper, zinc, or iron. Located in the active sites of enzymes, these ions attract electrons from substrate molecules. For
instance, carboxypeptidase breaks down proteins using a zinc cofactor. This cofactor draws electrons away from bonds, which causes them to break. If cofactors are organic, non-protein molecules, they are also called coenzymes. Many vitamins, small organic molecules that the human body requires in trace amounts to function, are parts of coenzymes.
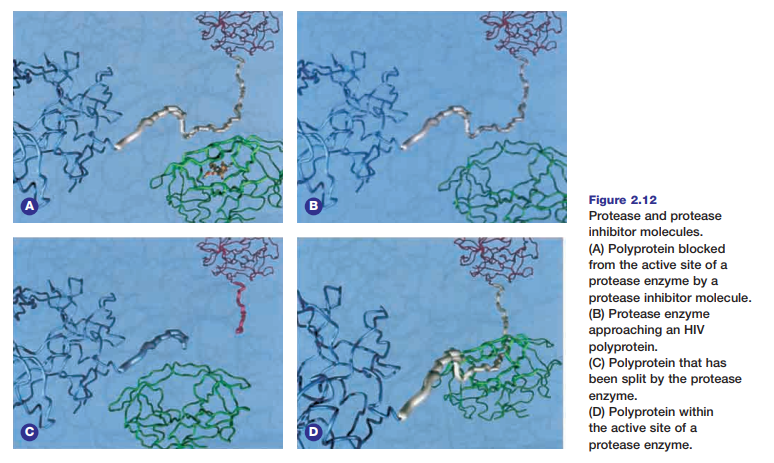
Table 2.3 shows vitamins necessary to the formation of specific coenzymes.
Deficiencies in any of these vitamins can affect the enzymatic reactions in cells. For example, lack of niacin may result in a lack of NAD+ (nicotinamide adenine dinucleotide), which can affect enzymatic reactions in cellular respiration. Niacin deficiency can cause a skin disease called pellagra. At one time this disease was often mistaken for leprosy, but in
the early 1900s American researcher Dr. Joseph Goldberger determined that pellagra is caused by a nutritional deficiency. To treat the disease, he recommended a diet that included meat, milk, fish, or a small portion of dried brewer’s yeast.
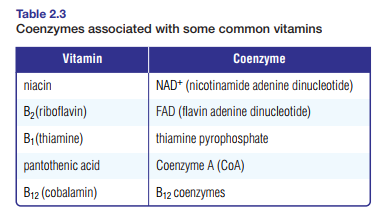
Both coenzymes NAD+ and FAD (flavin adenine dinucleotide) serve as electron acceptors in redox reactions. They carry electrons from one active site to another. Once the electrons have been released, the coenzymes return to the original enzyme for another complement of electrons.
The NAD+ coenzyme takes the energy from the oxidation of nutritive molecules digested by animals to form NADH, a molecule with more chemical energy. NADH is then oxidized into NAD+ again in order to collect more electrons.
NAD+ is the principal carrier of electrons in the oxidation of molecules that are used as an energy source in the cell. For example, NAD+ accepts electrons from the products of the breakdown of glucose in one stage of cellular metabolism, and then transports them to a metabolic pathway that reduces oxygen to water. During such reactions,
NAD+ accepts two electrons, but only one hydrogen ion, as shown in the following equation:
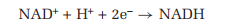
When NADH is oxidized back into NAD+, energy is released. Similar in function to NAD+, NADP+ (NAD+ plus an additional phosphate group) is a coenzyme in photosynthetic reactions.
Enzymes and Coenzymes for Human Health and Industry
Enzymes and coenzymes have proven useful in medical and industrial applications. Medical researchers have been conducting tests using NADH on patients with Alzheimer’s disease or Chronic Fatigue Syndrome (CFS). In a study conducted in the 1990s at Georgetown University Medical Center, CFS patients who received injections of NADH experienced only one quarter of the symptoms experienced by patients who were given a placebo (a substance with no medical value). At the end of the twentieth century, six out of 10 individuals who were taking NADH used it to
improve their energy level; two out of 10 used it to control Alzheimer’s symptoms; and one out of 10 took it to relieve CFS. Enzymes are also used in the process of DNA fingerprinting, which you will learn more about in Chapter 9. DNA fingerprinting has been used in a variety of circumstances, including paternity tests, murder trials, and identifying people. In one step of the DNA fingerprinting process, special enzymes called restriction enzymes are used to cut the
DNA at specific places. DNA restriction enzymes recognize short, specific sequences of DNA bases and make breaks in the sugar–phosphate backbone of the DNA molecule in the region of the recognized sequence. Without these enzymes, the process of DNA fingerprinting would be much more involved.
DNA fingerprinting also uses a process called PCR, polymerase chain reaction, which you will learn about in Chapter 9. DNA can play a role in determining whether or not an individual’s enzymes are functioning normally. For example, Hurler syndrome is a genetic disorder caused by a defective gene. A chil born with Hurler syndrome cannot anufacture
the enzyme alpha-L-iduronidase. This enzyme is one of 10 lysosomal enzymes responsible for breaking down complex carbohydrates called mucopolysaccharides (MPS). Mucopolysaccharides are largely responsible for building connective
tissues in the human body. If mucopolysaccharides cannot be broken down properly, they build up in body cells and form excess tissue. A child diagnosed with Hurler syndrome will become afflicted with various cardiac or respiratory ailments by the age of five and not survive long thereafter.
People have also found ways to exploit enzymes and coenzymes for industry and profit. One of the most obvious ways that enzymes can be used in industry is in wine-making. Before 1897, scientists believed that enzymes required living material to function. The first to discover that a cell-free, or non-living, extract of yeast could cause alcohol fermentation was the German chemist Eduard Buchner (shown in Figure 2.13). His experiments led to the use of enzymes in industries as diverse as wine production, leather tanning, food production, textiles, pulp and paper, and
pharmaceutical manufacturing.
Enzymes are essential to the pharmaceutical industry in making products — from chemotherapy treatments to common painkillers. Many of these products are composed of enzymes or make use of enzymatic reactions. As well, they often affect the activity of enzymes within the body. A new form of chemotherapy, Antibody-directed Enzyme Prodrug Therapy (ADEPT) uses enzymes to improve the efficiency of the drugs being used in the treatment of common solid tumours. This process involves using tumour-associated antibodies directed against tumour antigens. Doctors link the antibodies to enzymes and administer them to the patient. A prodrug is administered separately. A prodrug is an
inactive drug that is only converted into its active form in the body by metabolic activity. At the site of the tumour, the enzyme converts the prodrug into an active compound that is toxic to the tumour. Painkillers, for example, affect enzymes in order to relieve headaches, inflammation, or swollen tissues. Aspirin™ and similar painkillers reduce
inflammatory pain by inhibiting enzymes called cyclo-oxygenase (Cox) 1 and 2. Cox-1 is located in the stomach, protecting it from hydrochloric acid in the digestive juices. Cox-1 is also found in blood platelets, where it aids in clotting reactions. Cox-2 is produced in the skin or joints following inflammation. Cox-2 is necessary in catalyzing the
formation of prostaglandin E2 (PGE2), which increases the sensitivity of nerves to pain. Until recently, biochemists believed that inhibition of PGE2 at the site of inflammation accounted for both the anti-inflammatory and painkilling actions of Aspirin™ and similar painkillers. Although Cox-2 is produced at the inflamed site of the body, recent studies have shown that nerve cells in the spinal cord and brain also begin to produce it. This results in the production of PGE2 throughout the central nervous system. Biochemists revised their knowledge of how and where Aspirin™ works. Aspirin™ reduces inflammatory pain not only at the inflamed site but also in the entire central nervous system.
Because PGE2 increases nerve sensitivity to pain, its manufacture throughout the central nervous system accounts for the tenderness surrounding inflamed tissues. Researchers suspect that the presence of Cox-2 and PGE2 may explain why people with inflamed tissues experience aches and pains and even appetite loss and depression.
Recent studies on synthetic oligosaccharides carbohydrates composed of a relatively small number of monosaccharides) indicate they have great potential as therapeutic agents. These compounds, which interfere with carbohydrate– protein reactions, are difficult to create in the laboratory. However, a new technology has been discovered using glycosidases, which are produced by genetically altering DNA. These altered enzymes catalyze the synthesis but not the hydrolysis of oligosaccharides, making them easier and less expensive to construct. The altered enzymes have been termed glycosynthases and can be used to make anti-ulcer agents, therapeutic drugs for
middle-ear infections, and infant formula additives, to name a few. Dr. Stephen Withers, a scientist at the University of British Columbia, and his co-workers were the first to develop glycosynthases.
They continue to be in the forefront of developing new ways to use these enzymes and their substrates in industry and medicine. The “Canadians in Biology” profile on the previous page provides a more complete account of the work
accomplished by Dr. Withers and his team. By lowering the activation energy needed by cells to start metabolic reactions, enzymes allow biological systems to undertake necessary processes at the temperatures that exist inside the cell. People have learned a great deal about enzymes and taken them from such diverse sources as yeast and organisms living in hydrothermal vents in order to manufacture foods and pharmaceuticals. The following section discusses another aspect of enzyme function and metabolic reactions within cells — coupled reactions and the production of ATP.
Enzymes and Energy
In the summer of 2001, a forest fire that had been started by a lightning strike raged through Kootenay National Park in British Columbia. Park officials allowed the fire to progress because the area was scheduled for a prescribed burn. Fires are a natural part of forest ecology and are important in forest regeneration. For example, some species of pine, such as the jack pine, drop cones that need the heat from a fire to open them and release their seeds. The Kootenay fire quickly consumed the dry grasses
and trees; it soon spread beyond the area park officials could manage, threatening nearby communities.
Many firefighters risked their lives to control the spread of the flames. By the time the fire was contained and eventually extinguished, thousands of hectares of forest had burned.
The chemical reaction that occurred in the fire involved oxygen and the wood that formed the trees. While the forest fire was an example of a reaction that occurred with oxygen outside cells, reactions with oxygen also occur inside cells. Energy is necessary to perform all cellular reactions, including redox, hydrolysis, and condensation reactions. Enzymes aid reactions within cells. Enzymes are necessary because they speed up the synthesis of energy-rich molecules needed for cellular processes.
In this chapter, you will learn how chemical reactions within cells are used to make energy-rich molecules.
Energy from these molecules is used for various cellular processes. The bonds that hold atoms together store energy in molecules. This energy can be used by a cell to do work. You will explore various factors that influence how molecular bonds are formed and broken. You will also discover which molecules are involved in cellular processes and how the energy from one reaction can be used to drive another reaction.
Thermodynamics and Biology
Many reactions occur inside every cell. These reactions, collectively known as metabolism, have been at the centre of much scientific investigation. For example, manufacturers of dietary supplements for athletes seek to isolate chemicals that increase metabolic activity. Creatine phosphate is one such chemical — it is a nitrogenous molecule that is stored in muscle cells. Enhanced stores of creatine phosphate in muscles have been shown to increase muscle mass and efficiency. The compound was synthesized and used in the former Soviet Union by elite athletes in the 1960s to increase their metabolic activity and performance. What are metabolic reactions, and why are they important?
To understand this, you must first understand how energy flows through systems.

To survive, all living things require energy, which is the capacity for doing work. Energy comes in different forms. For instance, energy comes from the Sun as light, and thermal energy from a furnace can be used to heat a home. All moving objects, such as falling water and pistons in an internal combustion engine, have kinetic energy. Energy can also be stored as potential energy. A molecule of glucose has potential energy. The potential energy stored in the bonds of a molecule is called chemical energy. If a molecule of glucose is broken down into carbon dioxide and water, the energy released can be used to do work. If a phosphate group is removed from a molecule of ATP, the chemical energy can be used to fuel various cellular processes.
Energy continually flows through living and non-living systems. The study of this flow of energy is called thermodynamics. Physicists and chemists have studied thermodynamics since the days of Sir Isaac Newton. Biologists also apply thermodynamics when they study metabolic processes and the energy transformations that take place within living systems. Scientists use the term system to identify a process under study, and they refer to it in relation to the rest of the universe. For instance, a hot drink in a sealed vacuum bottle is considered a closed system because the liquid is isolated from its surroundings — thermal energy cannot move from the liquid to outside the bottle.
Removing the lid from the bottle results in an open system, because energy (thermal, in this case) can now move between the liquid and its surroundings — it moves from the liquid to outside the bottle. All living organisms are open systems; energy moves two ways, both in and out of cells. For example, a green plant absorbs energy from the Sun and uses this energy for building structures, transporting materials, growth, and reproduction. The plant also releases energy into the environment in the form of thermal energy when the plant is forming metabolic products, such as water and carbon dioxide.
How energy flows between organisms and the environment is governed by the laws of thermodynamics. You have already encountered these laws in previous studies. The first law, or law of conservation of energy, states that energy can neither be created nor destroyed, but can be transformed from one form to another. For example, during photosynthesis, a green plant absorbs light energy from the Sun. This energy is transformed into chemical energy, which is stored in bonds that hold together atoms in a molecule of sugar. An internal combustion engine converts the chemical energy stored in gasoline molecules into kinetic energy — the motion of the car. Some chemical reactions, such as burning a fuel, release energy. Some of this energy is useful because it is available to do work. The energy available to do work is known as free energy. Free energy can be used to do the work of building molecules in a cell. However, whenever energy is transformed from one form to another, some of it is lost. This lost energy is the portion that is not free energy and therefore is not available for useful work. The amount of free energy that can be harnessed by a green plant or car is much less than the total amount of light or chemical energy present in the sunlight or gasoline. This fact is the basis of the second law of thermodynamics, which states that energy cannot be transformed from one form to another without a loss of useful energy. The energy that is lost eventually escapes into the atmosphere largely as waste thermal energy. There are many transformations of energy that occur inside a cell.
During each transformation, some energy is lost as thermal energy. Eventually, all forms of useful energy are transformed into thermal energy. After thermal energy dissipates, it can never be transformed back into a useful form, such as chemical energy, that can be used to do work. Therefore, biological systems require a constant supply of energy from the Sun to function.
A measure of the tendency of a system to become unorganized is called entropy. Every transformation of energy creates more disorder in the universe. Therefore, we can restate the second law of thermodynamics as follows: every energy transformation increases the entropy of the universe.
The conversion of chemical energy into thermal energy does not violate the first law of thermodynamics. If thermal energy is produced during a chemical reaction, it is still a form of energy. Although some of this energy is not available to do work, energy is still conserved.
Consider the following example as a case study of thermodynamic principles. Stacked beside the fire pit at your campsite are a stack of newspapers and a bundle of kindling that you intend to ignite to start a fire. The stack of paper and the wood are composed of cellulose, which is made up of complex carbon-based molecules. These molecules contain potential chemical energy. When you light the paper, the chemical bonds in the molecules are broken in a reaction with oxygen. During the reaction, thermal energy and light are released.
Recall from your study of Chapter 1 that this is a redox reaction. Once the reaction begins, the paper quickly burns, forming the products of the oxidation of cellulose: carbon dioxide and water. If energy is released from the reaction of paper with oxygen, the paper and oxygen must contain more chemical energy than the products (see Figure 2.2).
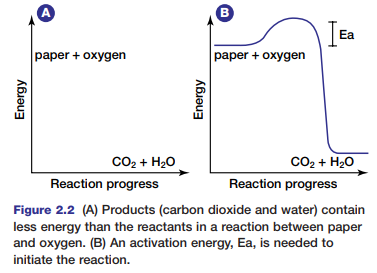
During the reaction, the chemical energy stored in the paper and in the oxygen molecules is transformed into thermal energy and light energy. You can feel the thermal energy that is released if you reach toward the fire to warm your hands. Why does the paper require an initial input of energy to start the fire? Chemical bonds hold atoms and molecules together. These bonds maintain the chemical energy in the molecules. In order to destabilize the bonds, and thereby release the energy they hold, an initial input of extra energy is needed. This extra energy is known as activation energy.
Figure 2.2 shows the activation energy required to ignite paper. Different substances require different amounts of activation energy to start a reaction. The activation energy needed to start a reaction within cells is governed by special proteins. Without these proteins, metabolic processes could not occur. Next, you will examine two types of metabolic reactions that occur within cells.
Exothermic and Endothermic Metabolic Reactions
Recall that metabolic reactions encompass all the reactions that occur within cells, including anabolic reactions (such as condensation) and catabolic reactions (such as hydrolysis), and redox reactions.
Complex carbohydrates, fats, and proteins can be broken down in catabolic reactions, thereby forming molecules such as simple sugars and amino acids. Anabolic processes then join up these products and their functional groups to form various macromolecules needed by cells for maintenance and growth. A reaction can be classified based on whether it releases or uses energy. A reaction that is accompanied by a release of energy is called an exothermic reaction, as shown in Figure 2.3 on the next page. For example, recall the overall reactionfor cellular respiration:
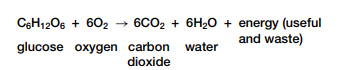
For each molecule of glucose oxidized in cellular respiration, energy is released. Some of this energy is useful and available to do work and some is waste thermal energy. This means that the products (carbon dioxide and water) contain less energy than the reactants (glucose and oxygen).
In contrast, an endothermic reaction involves an input of energy. For example, the synthesis of glucose
by plants during photosynthesis is as follows:

endothermic reaction stores chemical energy in molecules, there is a gain in energy.
As you can see in the two equations above, oxidation and synthesis of glucose are two reactions that are the reverse of each other. If two reactions are the reverse of each other, one reaction is endothermic and the other is exothermic. Exothermic and endothermic reactions both involve energy transformations. How do cells control the flow of energy so that they do not overheat and destroy themselves? In the next
section, you will learn how cells are able to lower the amount of activation energy necessary to carry out a variety of metabolic reactions.

Making and Breaking Macromolecules
Large molecules can be broken down to release energy. Alternatively, they can be formed to build cellular structures or store information. In biological systems there are four major types of chemical reactions involved in breaking apart and
building molecules: acid-base or neutralization reactions, which transfer hydrogen ions between molecules, redox, or oxidation-reduction reactions, which transfer electrons between molecules, hydrolysis reactions, in which molecules react with H2O to form other molecules, and condensation reactions, in which molecules react to form H2O and other molecules.
These types of chemical reactions are described below.
Acids, Bases, and Neutralization Reactions
Acids and bases are compounds that may be inorganic or organic. Hydrochloric acid, found in the mammalian stomach, is an inorganic acid. Acetic acid and amino acids are examples of organic acids. Sodium hydroxide, a key component of oven cleaners, is an inorganic base. Purines and pyrimidines, the molecules that form part of the
subunits of nucleic acids, are examples of organic bases; they are often referred to as nitrogenous bases, because they include the nitrogen-containing amine group. What is it, however, that makes one substance an acid and another a base? In biology, acids and bases are understood in relation to their behaviour in water. Under normal conditions, pure water exists in the form of H2O molecules. A small number of these molecules dissociate, which means that they
break up into ions. When a water molecule dissociates, it forms a positively charged hydrogen ion, H+, and a negatively charged hydroxide ion, OH-. Since very few water molecules dissociate, the concentration of these ions is low. In pure water at 25°C, the concentration of each of these ions is the same: 1 × 10-7 mol/L. Because hydrogen and
hydroxide ions are very reactive, changes in their concentrations can drastically affect cells and the macromolecules within them. Acids and bases, and more specifically the concentrations of hydrogen and hydroxide ions within cells, determine how effectively cellular processes are carried out.
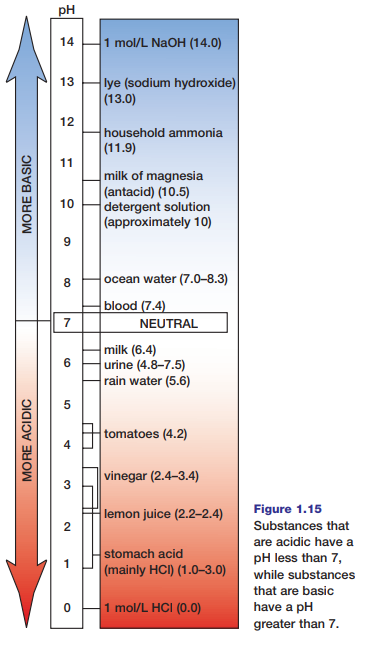
An acid is any substance that donates H+ ions when it dissolves or dissociates in water. Therefore, acids increase the concentration of H+ ions in water solutions. Bases, on the other hand, decrease the concentration of H+ ions in solution. Usually this occurs because bases attract H+ ions, thus reducing their concentration. As a result, the concentration of OH- ions increases when bases dissolve or dissociate in water. The pH scale, shown in Figure 1.15, is a means for ranking substances according to the relative concentrations of their hydrogen and hydroxide ions. Water, with equal concentrations of these ions, is considered neutral and has a pH of 7. Substances with a pH that is lower than 7 have higher concentrations of H+ ions (and lower concentrations of OH- ions), so they are acids. Substances with a pH that is higher than 7 have lower concentrations of H+ ions (and higher concentrations of OH- ions), so they are bases.
When acids and bases react, they produce two products: water and a salt (an ionic compound). This chemical process in which acids and bases react to product a salt and water is called a neutralization reaction. In such a reaction, the acid no longer acts as an acid and the base no longer acts as a base; their properties have been neutralized.
Buffers
Many biological processes require specific pH levels in order to function properly. For example, pH and the control of pH play an integral role in both photosynthesis and cellular respiration. Many proteins require a certain pH in order to take on their characteristic shapes. Therefore, it is important for pH in organisms to be maintained at specific levels. Certain chemicals or combinations of chemicals known as buffers minimize changes in pH. Buffers maintain pH levels by taking up or releasing hydrogen ions or hydroxyl ions in solution. You will investigate the effect of a buffer in living cells in the next investigation. In Chapter 4, you will see how buffers play an important role in maintaining blood pH.
Redox Reactions
Almost every element on Earth can react with oxygen. For instance, if oxygen combines with calcium, the oxygen receives electrons and forms negatively charged ions.
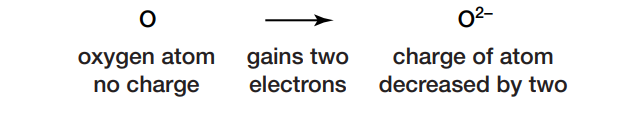
The addition of two electrons has decreased the charge of the oxygen atom by two. The gain of electrons is referred to as reduction. The calcium loses electrons and forms positively charged ions, as shown here:

The loss of electrons is called oxidation.
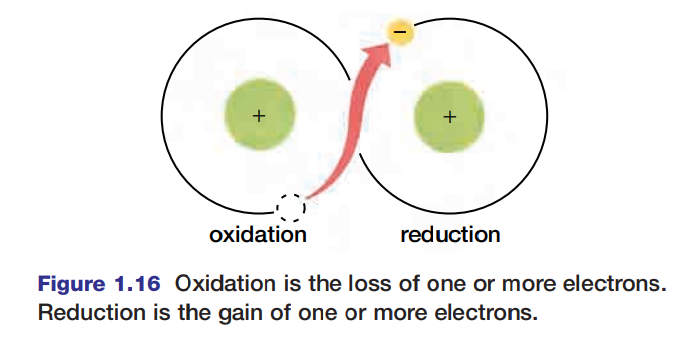
The terms “oxidation” and “reduction” are applied to many reactions involving ions whether or not oxygen is involved. For instance, in the reaction Na + Cl → NaCl, chlorine is reduced (gains an electron to form Cl-) and sodium is oxidized (Na loses an electron to form Na+). Because reduction and oxidation are both involved in the process, the entire reaction is called a redox reaction. Figure 1.16 is a generalized schematic representation of a redox reaction.
Cellular respiration is an important example of a redox reaction that takes place in biological systems. The overall reaction is:
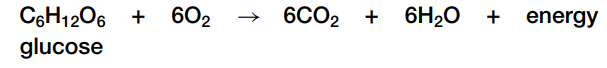
In cellular respiration, high-energy electrons are removed from food molecules, which oxidizes them. These high-energy electrons are transferred to increasingly electronegative atoms, and help the cell manufacture energy-rich molecules used by cells to do work.
Hydrolysis and Condensation Reactions
Macromolecules in living systems are built and broken down by hydrolysis and condensation reactions (see Figure 1.17). In condensation (or dehydration synthesis), the components of a water molecule are removed to bond two molecules together. Because the organic molecule formed is bigger than the two organic molecules that reacted,
condensation is an anabolic process. In the process of hydrolysis, the components of a water molecule are added to a molecule to break it into two molecules. Because the organic molecules produced are smaller than the organic molecule that reacted, hydrolysis is a catabolic process. Read on to see how hydrolysis and condensation work to
break down and build carbohydrates, nucleic acids, proteins, and lipids.
Making and Breaking Carbohydrates
Carbohydrates are important macromolecules because they store energy in all organisms. Carbohydrates are groupings of C, H, and O atoms, usually in a 1 : 2 : 1 ratio. Often, carbohydrates are represented by the chemical formula (CH2O)n, where n is the number of carbon atoms in the carbohydrate.
Carbohydrates can be simple, such as the monomer glucose. Glucose is a hexose (six-carbon) sugar with seven energy-storing C-H bonds. If the number of carbon atoms in a carbohydrate molecule is low (from three to seven), then it is a
monosaccharide. Greek prefixes for the numbers three through seven are used to name these sugars.
For example a five-carbon sugar is a pentose, and a six-carbon sugar is a hexose. The glucose, fructose, and galactose isomers you studied in the previous section are all hexoses. Glucose is the primary source of energy used by cells.
Two monosaccharides can bond to form a disaccharide. For example, two glucose molecules can join to form the disaccharide maltose, as shown in Figure 1.18.
Organisms store energy in molecules known as polysaccharides. Polysaccharide molecules, such as starch and glycogen, are polymers made up of chains of linked monosaccharides. The long chains of glucose molecules, which make up starch, glycogen, and some other polysaccharides, are formed by a condensation reaction, which removes water from 2 –OH functional groups or neighbouring monosaccharides. Because of its chemical composition, cellulose (a polysaccharide found in all plants) is indigestible for animals. The bonds in cellulose are difficult to break by normal metabolic means. In contrast, other polysaccharides, such as the amylopectin found in potatoes, rice, and wheat,
serve as convenient and accessible forms of stored energy. The bonds that bind their high-energy glucose molecules together are easily broken and easily formed.
In living cells and tissues, polysaccharides and disaccharides can be broken into smaller units by the process of hydrolysis. The complete hydrolysis of most forms of starch produces a form of glucose, which is a simple sugar that cannot be decomposed by hydrolysis. In the investigation on page 24, you can determine the products of hydrolysis reactions.
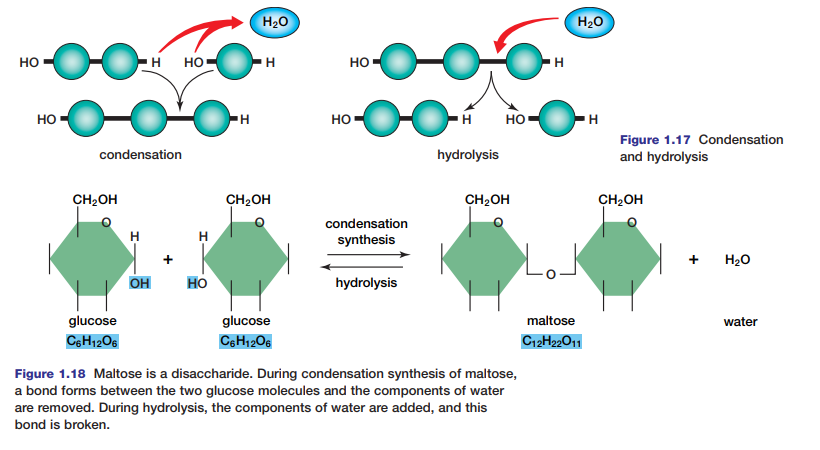
Nucleotides and Nucleic Acids
Nucleic acids such as DNA and RNA are huge polymers of nucleotides. These are molecules composed of one, two, or three phosphate groups, a five-carbon sugar (deoxyribose or ribose), and a nitrogen-base (see Figure 1.19). DNA contains genetic information about its own replication and the order in which amino acids are to be joined to
form a protein. RNA is the intermediary in the process of protein synthesis, conveying information from DNA regarding the amino acid sequence in a protein. There are four different bases in DNA — adenine, thymine, guanine, and cytosine. In RNA, uracil replaces thymine as a base. Adenine not only helps code genetic material and build proteins, but it also has important metabolic functions. You will investigate the structure and functions of nucleic acids further in Unit 3.
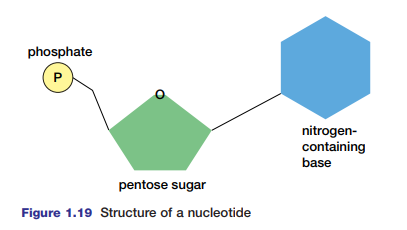
ATP, adenosine triphosphate, is composed of adenosine (adenine joined to ribose, as in RNA) and three phoshate groups (see Figure 1.20). The hydrolysis of ATP results in the formation of ADP and a phosphate (Pi), and in the release of a large quantity of energy for cellular work. After ATP breaks down, it can be rebuilt by the addition of the phosphate to ADP by condensation.
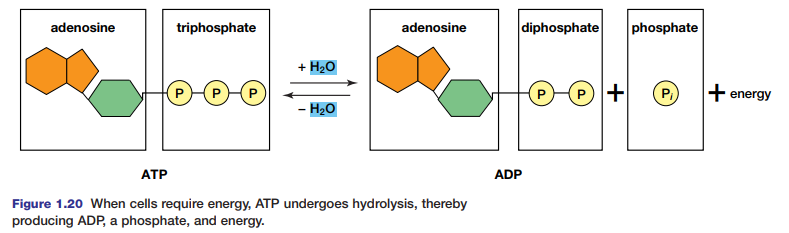
Condensation Synthesis and Hydrolysis of Proteins
Proteins are important as structural components, sources of nutrition, and for their role in speeding up metabolic processes in the cell. Peptide bonds formed in condensation reactions link amino acids in proteins (see Figure 1.21). Each amino acid is composed of a carbon atom bound to a hydrogen atom and three additional groups — an amino
group, -NH2, a carboxyl group, –COOH, and an R-group that is different in each amino acid. When two amino acids join, they become a dipeptide. A chain of amino acids is called a polypeptide. Try the Thinking Lab to model a polypeptide. Polypeptides may join to form proteins. The sequence of these polypeptides, their particular orientations in space, and their three-dimensional shapes determine the type of protein they form. Enzymes, essential to metabolism (as you will see in Chapter 2), are proteins that are shaped in different ways depending
on their function. Some proteins are composed of many polypeptides. These polypeptides can be broken during metabolism by hydrolysis.
Breaking Lipids
Lipids include fats and phospholipids (such as those in the cellular membrane), steroids, and terpenes (lipid pigments that operate during photosynthesis). Fats are composed of glycerol and three fatty acids; steroids and terpenes are composed of carbon rings and carbon chains respectively.
Fat is usually of animal origin and is solid at room temperature. Within animal bodies it is used for long-term energy storage. Fat also insulates against external heat and cold and protects major organs. Oil, the plant equivalent to fat, is liquid a room temperature. Fats and oils are often called triglycerides because of their structure. Fats and oils are insoluble in water because they are non-polar.
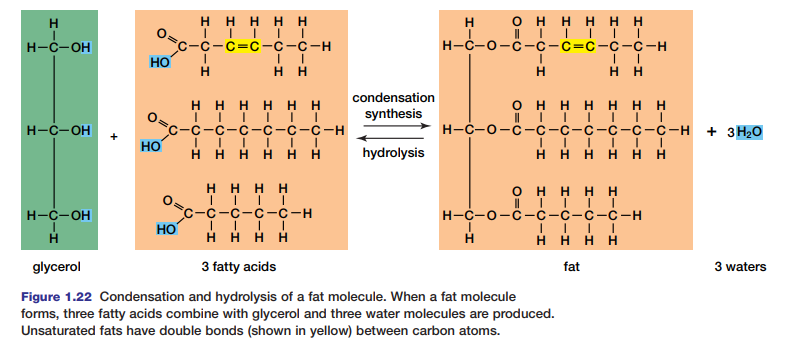
Both fats and oils are composed of two types of molecules: glycerol and fatty acids. Glycerol is a three-carbon alcohol in which each carbon is attached to a hydroxyl group (–OH), as shown in Figure 1.22. This three-carbon molecule is the
core of the fat or oil molecule. In a condensation reaction, three fatty acids are attached to this core to form a fat. A fatty acid is a hydrocarbon chain that ends with the carboxyl group (–COOH). Most of the fatty acids in cells contain 16 or 18 carbon atoms per molecule. Saturated fatty acids have no double bonds between their carbon atoms; the carbon chain is “saturated” with as many hydrogen
atoms as it can hold. Saturated fatty acids are generally solid at room temperature. In contrast, unsaturated fatty acids have one or more double bonds between carbon atoms. Therefore, the fatty acid is not saturated with hydrogen atoms. Fat molecules are split by hydrolysis for use in cells.
Figure 1.23(a) shows another lipid macromolecule with a different function in cells.
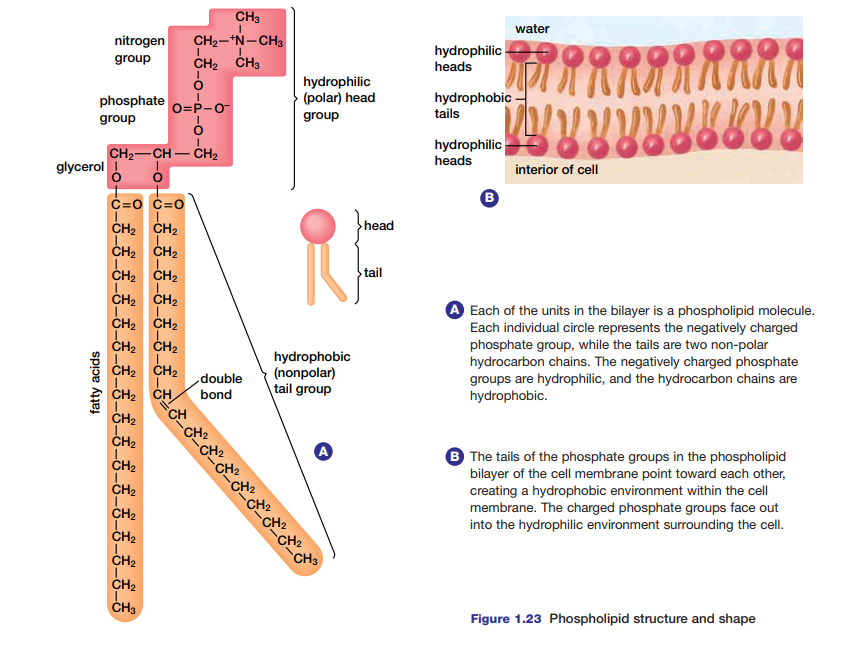
Called a phospholipid, this molecule interacts with water in a way that spontaneously results in the structure shown in Figure 1.23(b). This phospholipid bilayer is the foundation for the semi-permeable membrane that surrounds cells.
Some molecules can pass freely through the membrane, while others require assistance to enter. The phospholipid bilayer is virtually impermeable to macromolecules, relatively impermeable to charged ions, and quite permeable to small, lipidsoluble molecules. Molecules that move through the membrane do so at differing rates, depending
on their ability to enter the hydrophobic interior of the membrane bilayer.
Many small, non-polar solute molecules, such as oxygen and carbon dioxide, pass through the bilayer of the cell membrane with least resistance. They enter by means of diffusion, a form of passive transport. As you learned in previous studies, in this method of cellular transport, molecules move from regions of high concentration to those of low concentration. Water, a small polar molecule, can travel through the cell membrane freely in the process of osmosis. This process involves the movement of the solvent water from an area of higher concentration of water to an area of lower concentration of water.
Some molecules are too large to diffuse unassisted across the cell membrane. These molecules enter the cell by means of specialized proteins called carrier proteins — they move and change shape to create an opening into the cell.
Large uncharged hydrophilic molecules such as glucose make use of these proteins in order to enter cells (see Figure 1.24). No cellular energy is required for this facilitated diffusion process, so it is a form of passive transport. Appendix 5 shows several other examples of passive transport through the cell membrane. In the next chapter, you will see how cells use energy to move larger molecules across the cell membrane.

Making and Breaking Macromolecules
Large molecules can be broken down to release
energy. Alternatively, they can be formed to build
cellular structures or store information. In
biological systems there are four major types of
chemical reactions involved in breaking apart and
building molecules:
acid-base or neutralization reactions, which
transfer hydrogen ions between molecules,
redox, or oxidation-reduction reactions, which
transfer electrons between molecules,
hydrolysis reactions, in which molecules react
with H2O to form other molecules, and
condensation reactions, in which molecules react
to form H2O and other molecules.
These types of chemical reactions are described
below.
Biological Macromolecules and Their Subunits
The atoms of four elements make up roughly 99 percent of the mass of most cells: hydrogen, nitrogen, carbon, and oxygen. With only a few exceptions, molecules that contain carbon atoms are called organic compounds. There are millions of different organic compounds. Nearly all organic compounds contain hydrogen as well as carbon, and most of these also include oxygen. Pure carbon and carbon compounds that lack hydrogen — such as carbon dioxide and calcium carbonate — are considered inorganic. Inorganic compounds are, nevertheless, integral components of living systems. See Figure 1.9. For example, water — an inorganic compound — provides a medium in which various substances may be dissolved and transported within and between cells.

Figure 1.9 In what ways do living and non-living systems,
and organic and inorganic compounds interact?
The Central Atom: Carbon
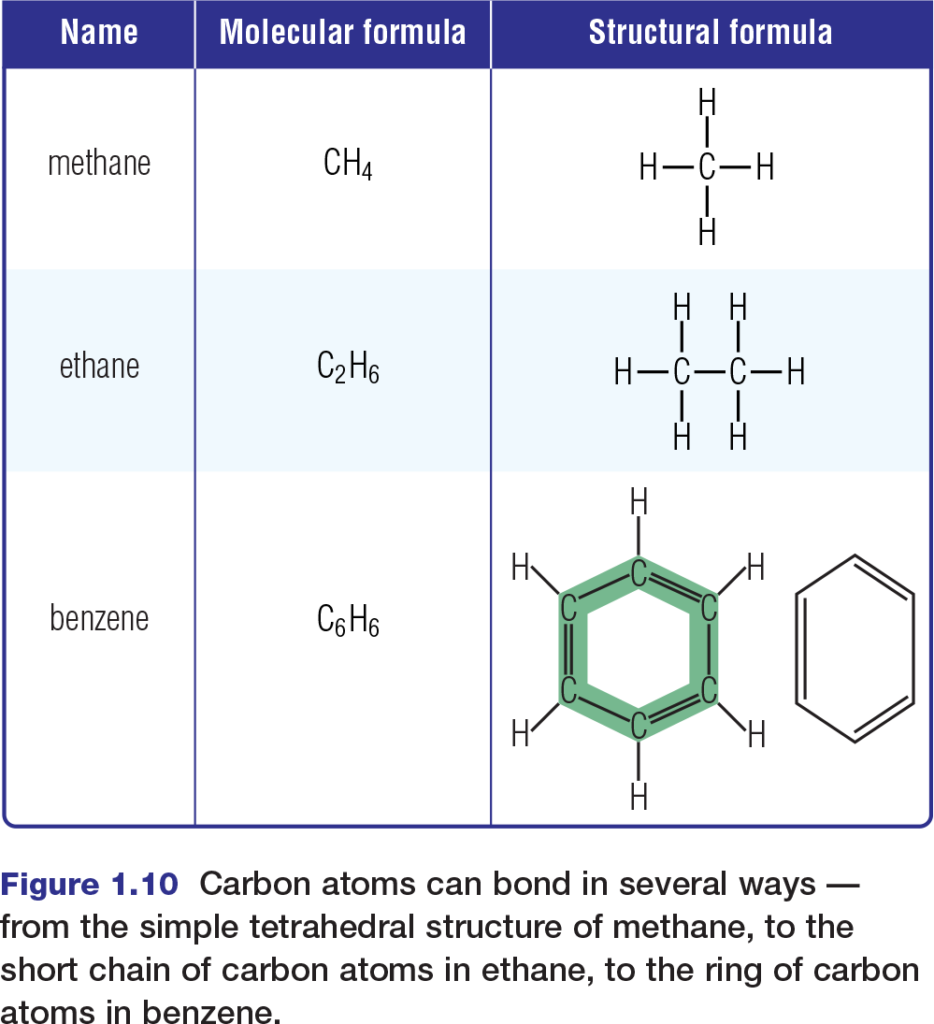
The diversity of life relies greatly upon the versatility of carbon. Recall that a carbon atom in its most stable state has two occupied energy levels, the second of which contains four valence electrons. This means that, in covalent molecules, a carbon atom can form bonds with as many as four other atoms. In biological systems, these atoms are mainly hydrogen, oxygen, nitrogen, phosphorus, sulfur, and — importantly — carbon itself. Carbon’s ability to bond covalently with other carbon atoms enables carbon to form a variety of geometrical structures, including straight chains, branched chains, and rings. Figure 1.10 shows the shapes of several simple organic molecules that contain only carbon and hydrogen atoms. These molecules, called hydrocarbons, comprise the fossil fuels that serve as the main fuel source for much of the world’s industrial activities. Hydrocarbons are themselves not components of living systems. However, substantial portions of many biological molecules consist of bonded chains of carbon and hydrogen.
Molecular Isomers
Because carbon can form so many compounds with so many elements, it is common to encounter several organic compounds with the same molecular formula but different structures. Such compounds are known as isomers. For example, two isomers of glucose, a six-carbon sugar, are fructose and galactose. Glucose, fructose, and galactose all have the same molecular formula (C6H12O6). However, they differ in their molecular structures, as shown in Figure 1.11.
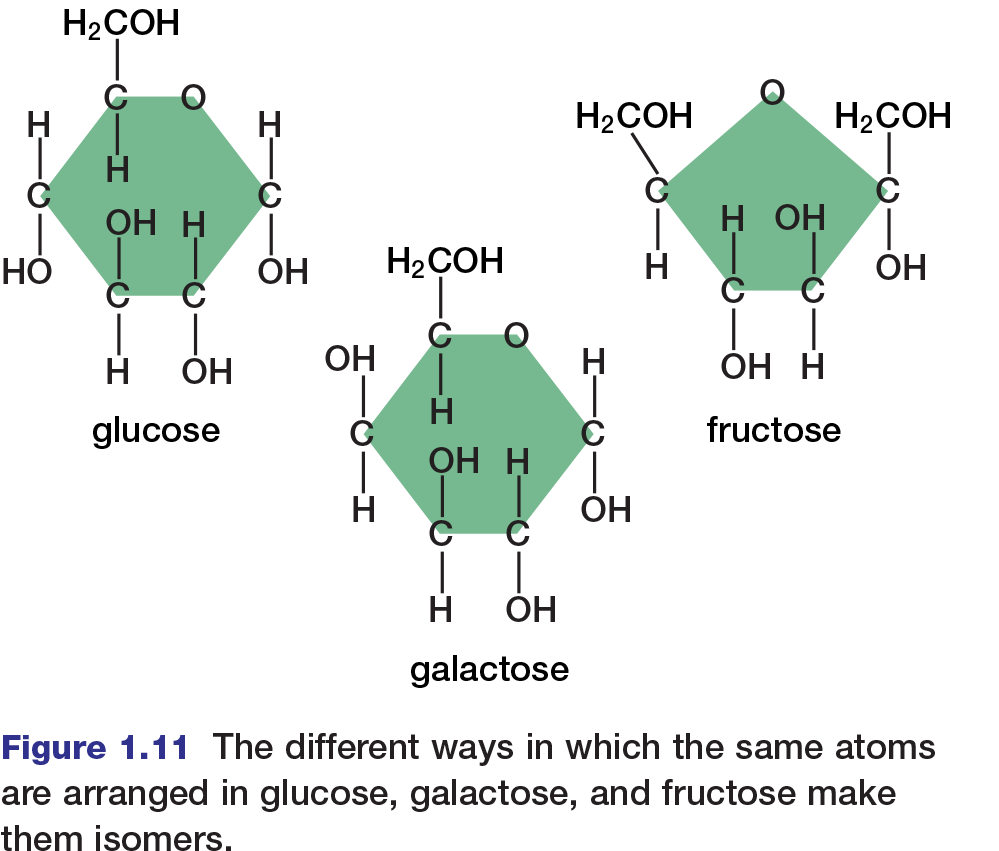
There are two main types of isomers. Structural isomers are two or more compounds with the same atoms bonded differently. Glucose and fructose, for example, are structural isomers. Notice that a glucose molecule contains a ring of five carbon atoms and an oxygen atom, whereas a fructose molecule contains a ring of four carbon atoms and an oxygen atom. Because their structures are different, glucose and fructose have different properties, and cells metabolize them differently.
Stereoisomers are two or more compounds with their atoms bonded in the same way, but with atoms arranged differently in space. Stereoisomers may be geometrical or optical. Geometrical isomers can have very different physical properties (such as different melting points), but they tend to have the same chemical properties. Glucose and galactose are examples of geometrical isomers.
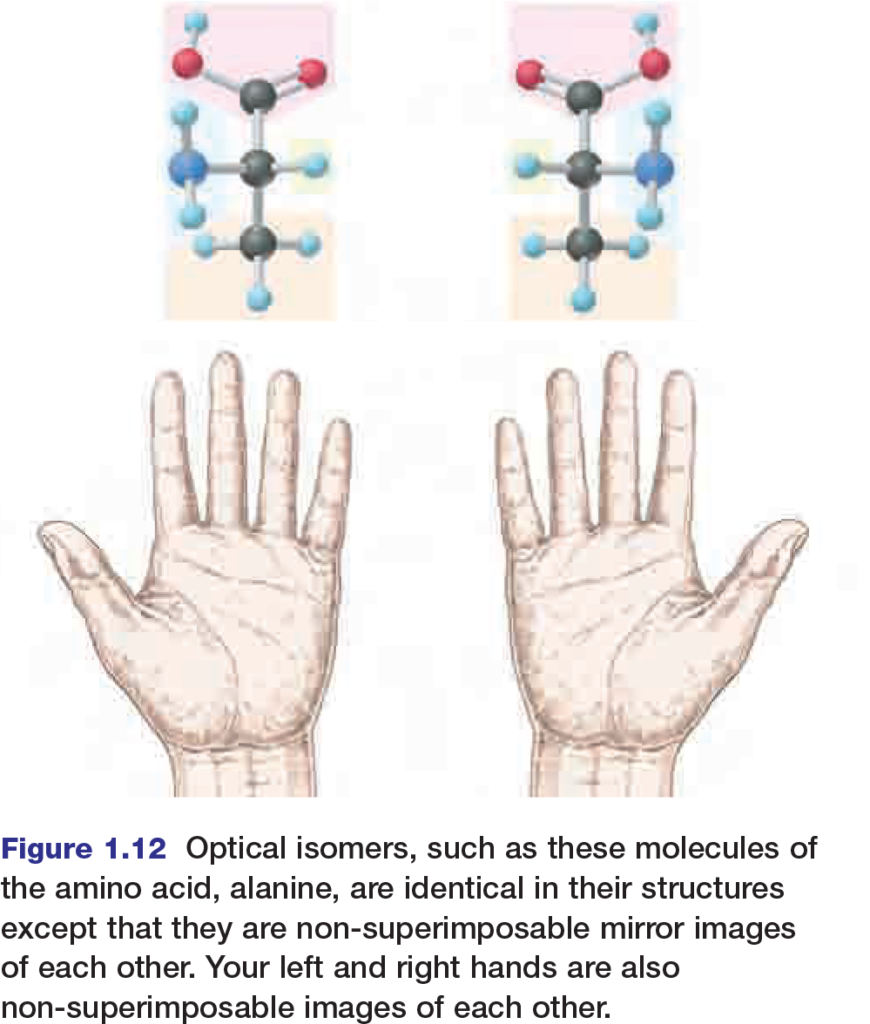
Optical isomers, shown in Figure 1.12, are nonsuperimposable mirror images of each other. They usually have similar chemical and physical properties, but enzymes or proteins on the cell membrane can distinguish between them. Usually, one optical isomer is biologically active and the other biologically inactive. In some cases however, this is not always true. For example, sometimes one optical isomer of a drug is not as effective as the other or can even cause complications. In the early 1960s, many pregnant women were prescribed a drug called thalidomide for morning sickness.
Thalidomide is a mixture of two optical isomers; one produced the desired effect, but the other caused major birth defects. As the thalidomide example demonstrates, organisms can be very sensitive to minute variations in molecular geometry.
The Functional Groups
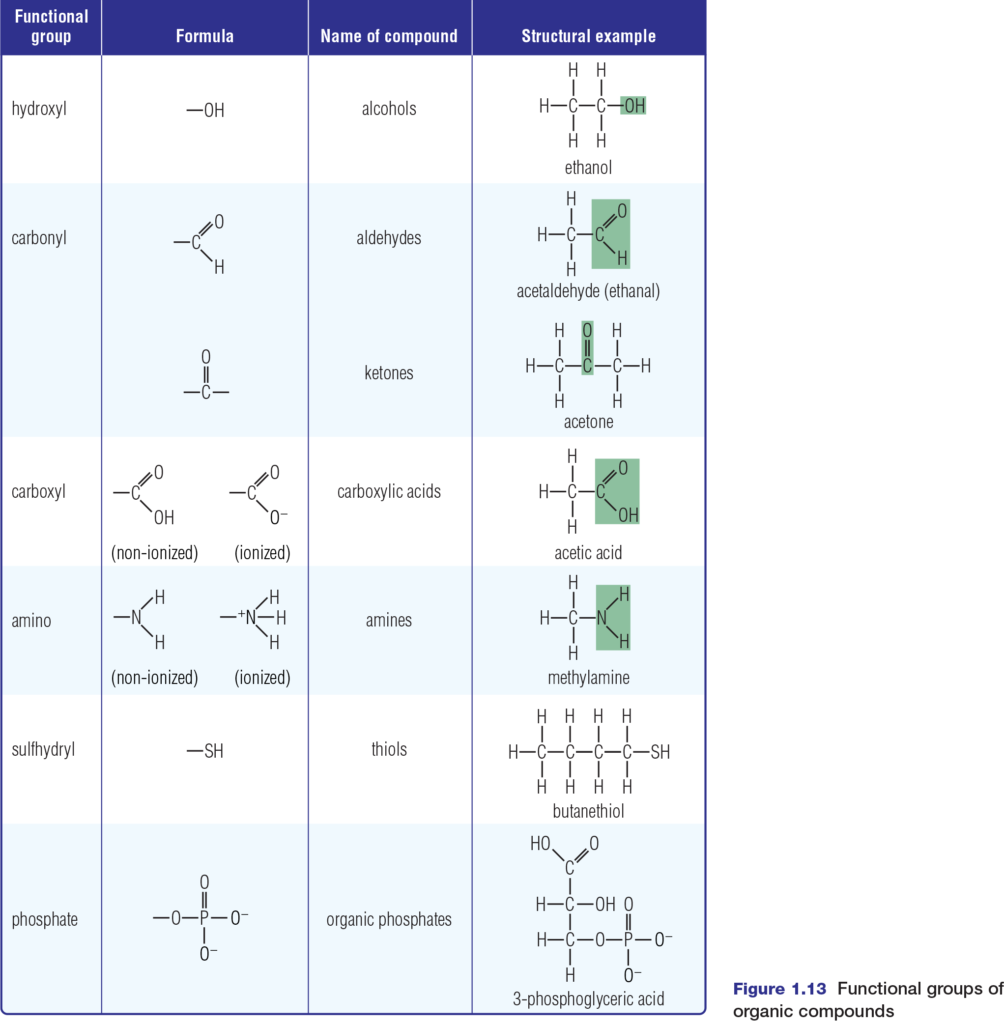
Chemical reactions involve breaking or forming chemical bonds. These processes can transform simple molecules such as glucose into complex molecules such as starch or cellulose. Many of these complex molecules contain groups of atoms with characteristic chemical properties. These groups of atoms, known as functional groups, include hydroxyl, carbonyl, carboxyl, amino, sulfhydryl, and phosphate groups, as shown in Figure 1.13 Many compounds have more than one functional group in their structure.
These functional groups are hydrophilic. Except for the phosphate group, they are polar and so they increase the solubility in water of the organic molecules to which they are attached. Each functional group also has capabilities to change the chemical properties of the organic molecules to which it bonds. For example, if a hydrogen atom in
ethane is replaced by a sulfhydryl group, the result is ethanethiol, also known as ethyl mercaptan. While ethanethiol in small amounts stabilizes protein structures, it is also a dangerous neurotoxin and respiratory toxin. Each functional group has a specific role in cell metabolism. Phosphates are essential to the metabolic processes of photosynthesis and cellular respiration. For example, the transfer of a phosphate group from ATP (adenosine triphosphate) begins the very important process of glycolysis — the first step in cellular respiration. You will discover more about this process in Chapter 3.
While amino and phosphate groups contribute to energy transactions in the cell, the sulfhydryl (–SH) group is essential to protein stabilization. Amino acids with –SH groups form bonds called disulfide bridges (S–S bonds) that help protein molecules to take on and maintain a specific shape.
Monomers and Macromolecules
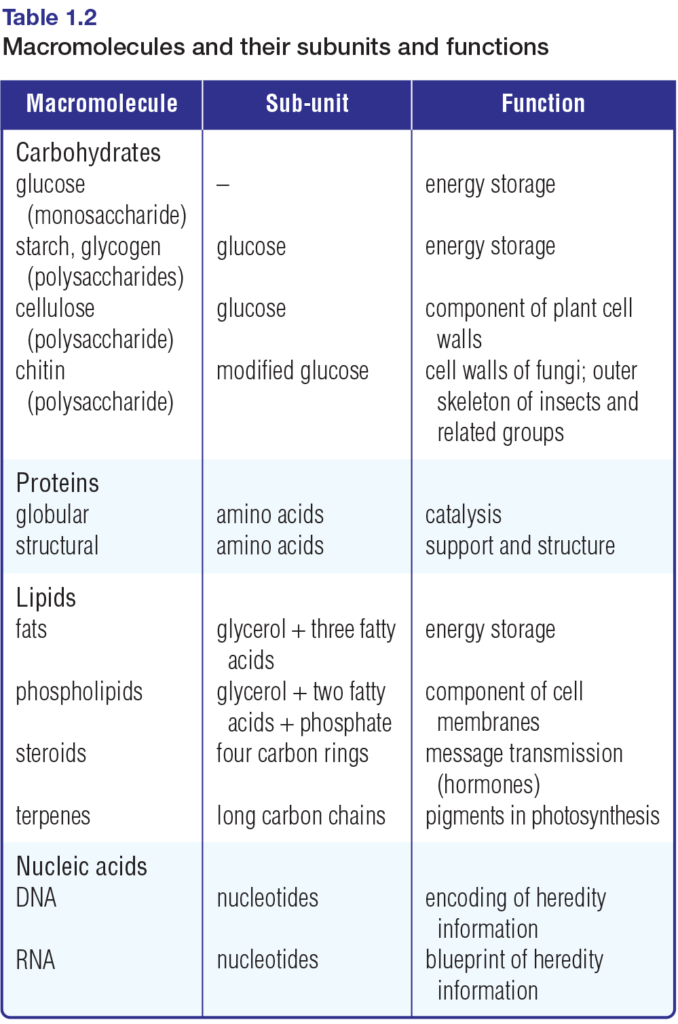
As you know, atoms can join together — bond — to form small compounds called molecules. Similarly, molecules can join together to form large structures called macromolecules. The small, molecular subunits that make up macromolecules are called monomers. The macromolecules themselves are built up of long chains of monomers. These chains are called polymers.
Table 1.2 lists the main types of macromolecules and their monomer subunits. Figure 1.14 depicts the subunits that comprise carbohydrates, selected lipids, proteins, and nucleic acids. Chemical reactions in cells synthesize macromolecules from these subunits, and break the molecules apart to release their subunits. Refer to Figure 1.14 often as you examine these chemical reactions in the final
section of this chapter.
Atoms and Bonding
Living things are unique among all forms of matter.
Unlike non-living things, all living things — from single-celled organisms such as the Euglena in Figure 1.1 to multicelled organisms such as whales and redwood trees — interact with and manipulate matter and energy. For example, all cells take in essential substances such as oxygen, water, and nutrients from their external environment. Inside cells, these substances undergo chemical reactions of several types. These reactions may be used to
break down substances, synthesize others, and repair defective structures. Chemical reactions also provide energy for these life-sustaining activities, as well as others such as reproduction. Unneeded (or harmful) products of the reactions are eliminated as wastes.
Collectively, these processes — intake of substances, processing of substances, and elimination of wastes — are called metabolic processes, or metabolism. The substances involved in metabolism are molecules. The bonds that form
between atoms define the structure and properties of these molecules. In this section, you will review several key ideas about atoms and bonding.
Figure 1.1 Euglena, a unicellular freshwater organism, carries out the same metabolic processes that your cells do.
Atoms and Elements
As you have learned in previous studies, all matter is formed of atoms. The atom is the smallest unit of matter involved in chemical reactions. Although tiny, atoms are complex structures, composed of even smaller subatomic particles. Most students of chemistry still study the model of the atom that Danish physicist Niels Bohr presented in the early twentieth century (see Figure 1.2). In this model, an atom consists of a small, dense core called a nucleus. It is composed of two kinds of subatomic particles — the positively (+) charged protons and the uncharged, or neutral, neutrons. Also in the Bohr model, negatively (−) charged electrons orbit the nucleus in one or more energy levels, or shells.
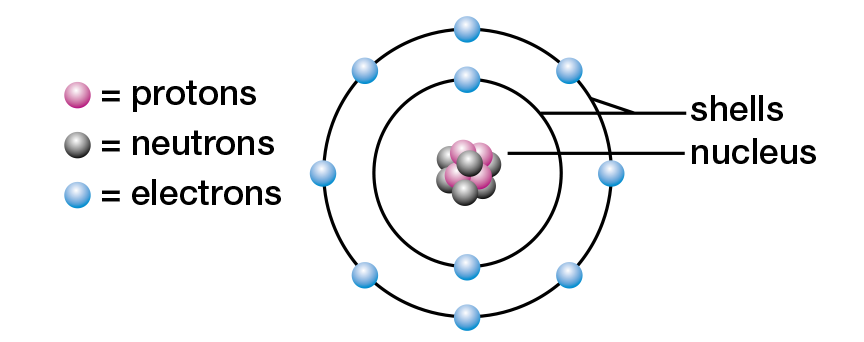
Figure 1.2 Niels Bohr’s model of the neon atom
An element is a substance that cannot be broken down into simpler substances by chemical means. Substances such as calcium, oxygen, potassium, iron, and carbon are all elements. A few elements, such as helium, occur as single atoms. Several elements, such as hydrogen, nitrogen, and oxygen, occur as molecules made up of two atoms. Such molecules are called diatomic. Other elements such as phosphorus and sulfur occur as molecules made up of more than two atoms.
All atoms of an element have the same number of protons in their nuclei. This number, called the atomic number, is different for every element. The nuclei of carbon atoms, for example, each contain six protons. Because the nuclei of most atoms also contain neutrons, another important characteristic of an atom is its mass number. The mass number of an atom is the total number of protons and neutrons in its nucleus. Atoms of the same element that contain different numbers of neutrons are called isotopes of that element. Refer to Figure 1.3 to see the numbers of protons, neutrons, and electrons in three isotopes of carbon. Their names include the mass number of each isotope: carbon-12, carbon-13, and carbon-14.

Figure 1.3 Carbon, one of the most important elements in living matter, has three naturally occurring isotopes. The
nucleus of each isotope contains 6 protons, but the number of neutrons in the nucleus is 6, 7, or 8. In each isotope,
6 electrons exist outside the nucleus.
Some isotopes are stable, whereas others are unstable and break down (decay). The unstable isotopes are known as radioactive isotopes.
Carbon-12 and carbon-13 are both stable isotopes, whereas carbon-14 is unstable and decays. Many radioactive isotopes decay at known rates. The rate at which a radioactive isotope decays may be used scientifically. The decay of carbon-14 can be used by archeologists, in a process called radiocarbon dating, to find the ages of some objects up to about 50 000 years old.
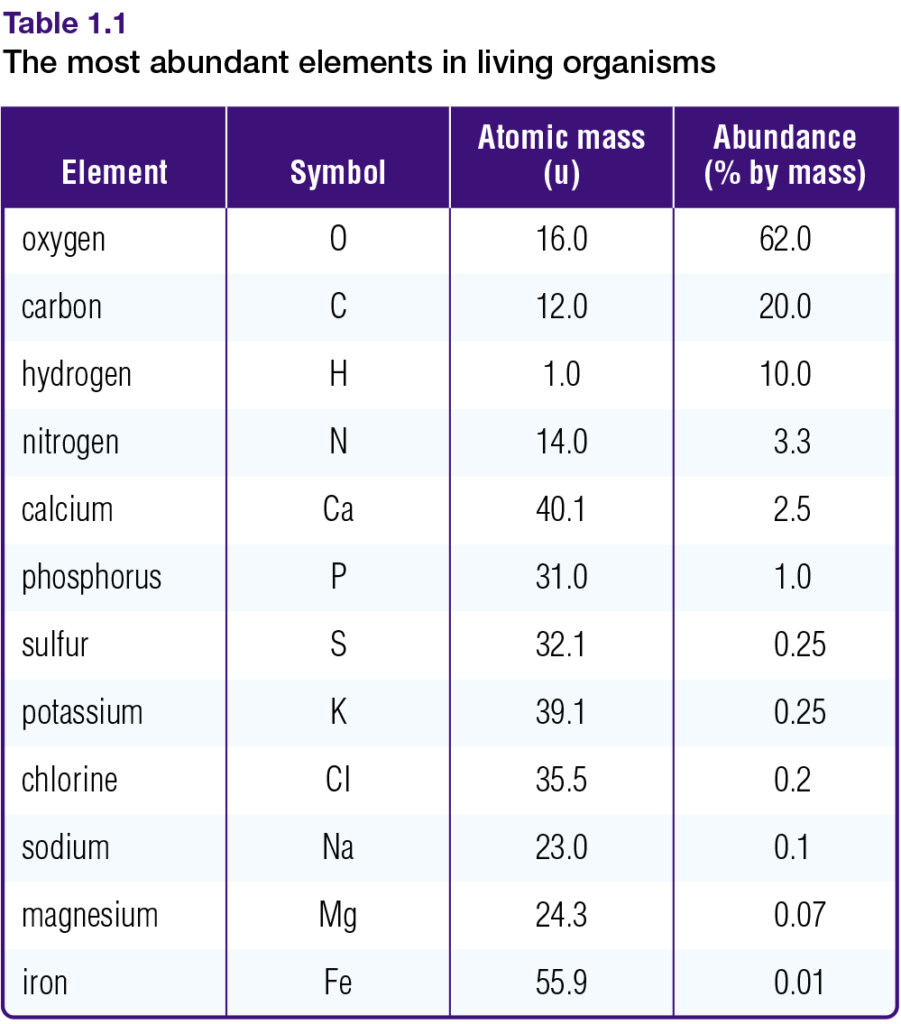
Table 1.1 shows the atomic masses of the elements that are most abundant in living organisms. Notice that, unlike atomic numbers and mass numbers, some atomic masses are not whole numbers. This is the case because the atomic mass of an element is the average mass of all the naturally occurring isotopes of that element.
Chlorine, for example, naturally occurs as a mixture of two isotopes: chlorine-35 and chlorine- 37. There are three chlorine-35 atoms for every chlorine-37 atom. Therefore, the average mass of chlorine atoms is closer to 35 than to 37. The atomic mass of chlorine is, in fact, 35.5 u (atomic mass units). Appendix 7 provides atomic masses for all the known elements.
Electron Energy
Biologists usually study the groups of atoms that make up molecules rather than atoms and subatomic particles themselves. All cells obtain the energy to function from chemical reactions that involve molecules. The actions of electrons are key to this process.
According to the Bohr model, electrons orbit the nucleus of an atom within energy levels, or shells. An electron in the first shell (nearest the nucleus) has the lowest amount of potential energy. Any electrons in the remaining shells have more potential energy. Each shell can hold a maximum number of electrons. The first shell, for example, can hold a maximum of two electrons, while the second shell can hold a maximum of eight. Refer to Figure 1.2, which shows that in a neon atom the first two shells are filled. In general, the maximum number of electrons that a shell can hold is given
by the formula 2n2, where n is the number of the shell. For example, the third shell can hold a total of 2(3)2 = 18 electrons.
The chemical properties of atoms rely mostly on the number of electrons in the outermost, occupied shell of an atom in its lowest energy state. This shell is known as the valence shell. The electrons that occupy the valence shell of any atom are called valence electrons. The elements in the periodic table that are least reactive are the noble gases, such as neon, found in group 18(8A) (see Appendix 7). Atoms of the other elements in the periodic table are more reactive than the noble gases. These elements can form chemical bonds with each other. The MiniLab examines safety issues involving the use of chemicals and how they react with each other during chemical bonding.
Ionic and Covalent Bonds
Most atoms can form chemical bonds with other atoms. These bonds are the forces that hold the atoms together in the form of compounds. For example, two chlorine atoms can combine (chemically react) to form a diatomic molecule of the element chlorine (Cl2). Atoms of sodium and chlorine can combine to form the ionic compound sodium chloride (NaCl).
There are two general types of chemical bonds. One type involves the sharing of electrons between atoms, and is known as a covalent bond. The other type involves the transferring of one or more electrons from one atom to another, and is called an ionic bond. How are these bonds formed?
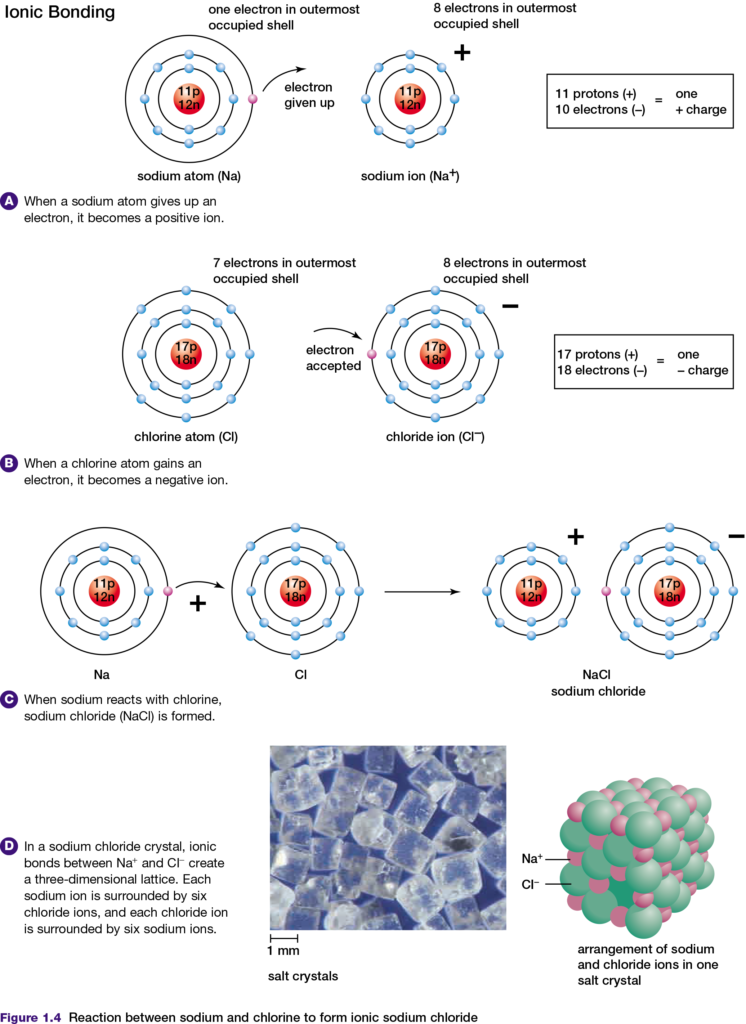
Any atom has the same number of electrons and protons. Therefore, the atom has no charge and is said to be neutral. However, if an atom loses or gains electrons, that atom becomes an ion. If an atom loses electrons, the ion formed has more protons than electrons and therefore has a positive charge. A positively charged ion is called a cation.
In contrast, if an atom gains one or more electrons the ion formed has a negative charge. A negatively charged ion is called an anion. When sodium (Na) and chlorine (Cl) atoms react, they form an ionic bond, as shown in Figure 1.4. The sodium atom gives up its only valence electron and becomes a sodium ion, with 11 protons and 10 electrons. This
number of electrons is arranged in the same way that the 10 electrons are arranged in the neon atom.
The chlorine atom gains an electron and becomes a chloride ion, with 17 protons and 18 electrons. This number of electrons is arranged in the same way as the 18 electrons in an argon (Ar) atom. Because the sodium ion is positively charged and the chloride ion is negatively charged, they attract each other to form an ionic bond. The tendency of chlorine to gain electrons is characteristic of atoms with a few electrons less than a noble gas atom. For example, atoms of fluorine and oxygen also tend to gain electrons when they form ionic bonds. One way to understand which elements form ionic bonds when they react is to use the principle of electronegativity.
Electronegativity is a measure of the relative abilities of bonding atoms to attract electrons. The Pauling scale is the most commonly used measure of electronegativities of atoms. Fluorine, the most electronegative element, is found near the top right corner of the periodic table and has an electronegativity value of 4.0. Both cesium and francium, the least electronegative elements, are found near the bottom left corner of the periodic table and each has an electronegativity value of 0.7. Elements that are most likely to form ionic bonds, such as sodium and chlorine, are far apart in the periodic table and have a large difference in their electronegativity. Elements that are close together in the
periodic table have a small difference in their electronegativity. If two of these elements react to form a compound, their similar abilities to attract electrons results in the formation of a covalent bond, in which electrons are shared. In a covalent bond, atoms share two valence electrons. An example of this is the covalent bonding of two chlorine atoms, as shown in Figure 1.5, top. Double covalent bonds involve the sharing of two pairs of shared valence electrons. The two oxygen atoms in an oxygen molecule are joined by a double covalent bond, as shown in Figure 1.5, middle.
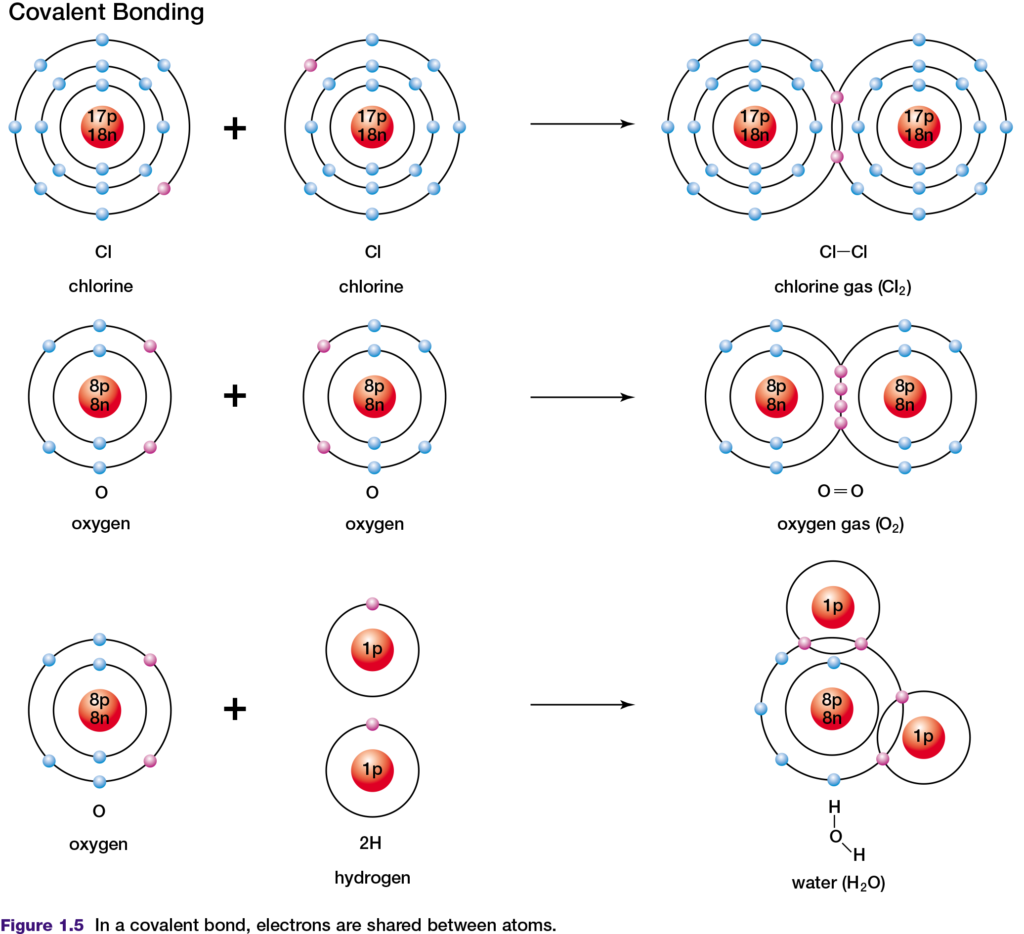
The shared electrons in covalent bonds belong exclusively to neither one nor the other atom. However, by sharing these valence electrons, both atoms appear to have the same number of valence electrons as a noble gas atom. In a covalent bond formed by two atoms of the same element, the electronegativity difference is zero. Therefore, the
electrons in the bond are shared equally between the two atoms. This type of bond is described as non-polar covalent. Examples of non-polar covalent bonds are found in chlorine and carbon dioxide molecules. A covalent bond is said to be polar covalent when the electronegativity difference between the atoms is not zero and the electrons are therefore shared unequally. In a water molecule (see Figure 1.5, bottom), oxygen is more electronegative than is hydrogen. The shared electrons spend more of their time near the oxygen nucleus than near the hydrogen nucleus. As a result, the oxygen atom gains a slight negative charge and the hydrogen atoms become slightly positively charged.
Chemists represent molecules formed through covalent bonds with various formulas, such as those in Figure 1.6. Electron-dot and structural formulas are simplified ways of showing what electrons are being shared.
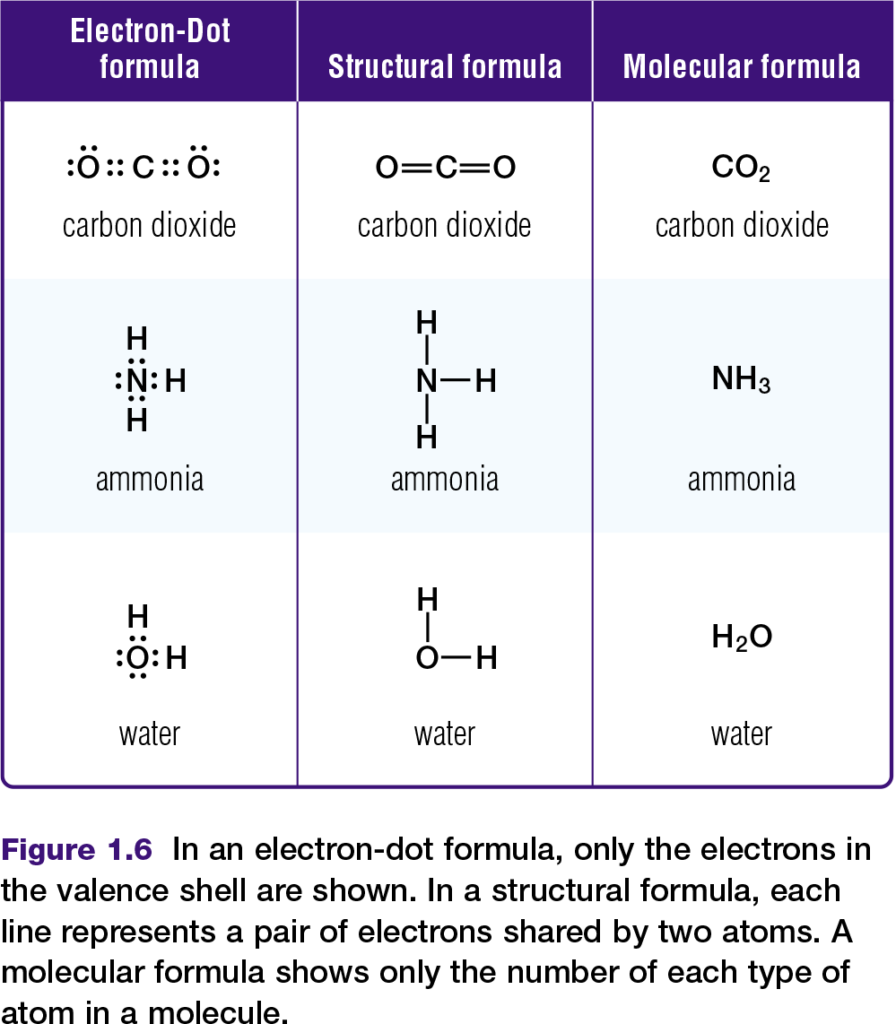
Hydrogen bonds and the properties of water
Some molecules with polar covalent bonds are known as polar molecules. A polar molecule has an unequal distribution of charge as a result of its polar bonds and its shape. More information about polar molecules is provided in Appendix 6. Water is a common example of a polar molecule. In a water molecule, as shown in Figure 1.7, the slightly negative end of each bond can be labelled δ− and the slightly positive end can be labelled δ+. These two ends, with slightly different charges, are sometimes referred to as “poles.” Because a water molecule is polar, it can attract other water molecules, due to the attraction between negative poles and positive poles (see Figure 1.7). The attractions between water molecules are called hydrogen bonds. Hydrogen bonds can also be found between other molecules that contain hydrogen atoms bonded covalently to atoms of a much more electronegative element. Examples include ammonia (NH3) and hydrogen fluoride (HF) in their liquid states.
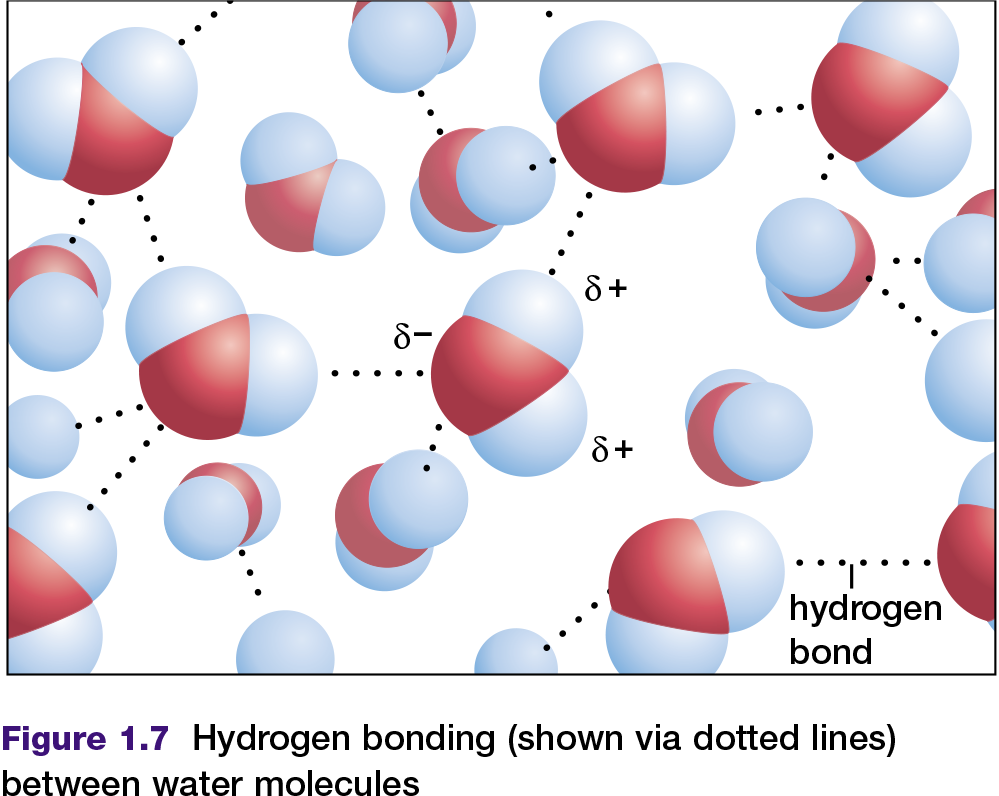
A hydrogen bond is a force between molecules, not a chemical bond within a molecule. Hydrogen bonds are usually weaker than chemical bonds. For instance, a hydrogen bond may be only five percent the strength of a covalent bond, but it is sufficient to hold one water molecule to another in liquid water or ice. Under normal conditions, water molecules are attracted to each other in such a way that they are neither attracted too strongly (to form a solid) nor too weakly (allowing water to become a vapour). For this reason, under normal conditions on Earth, water exists as a liquid.
Solubility of Substances in Water
All cells depend on liquid water. In fact, living organisms contain more molecules of water than any other substance; water comprises as much as 90 percent of a typical cell. Water is a perfect fluid environment through which other molecules can move and interact. Sodium chloride (table salt), and many other ionic compounds or salts, dissolve readily in water. This occurs because the positively charged poles of the water molecule are attracted to the anions
(chloride ions) in the salt. The negatively charged pole of the water molecule is similarly attracted to the cations (sodium ions) in the salt as shown in Figure 1.8. These two attractions pull the sodium ions and chloride ions away from each other. The salt is now dissociated, which means that the sodium ions and chloride ions have separated and have dissolved in the water.
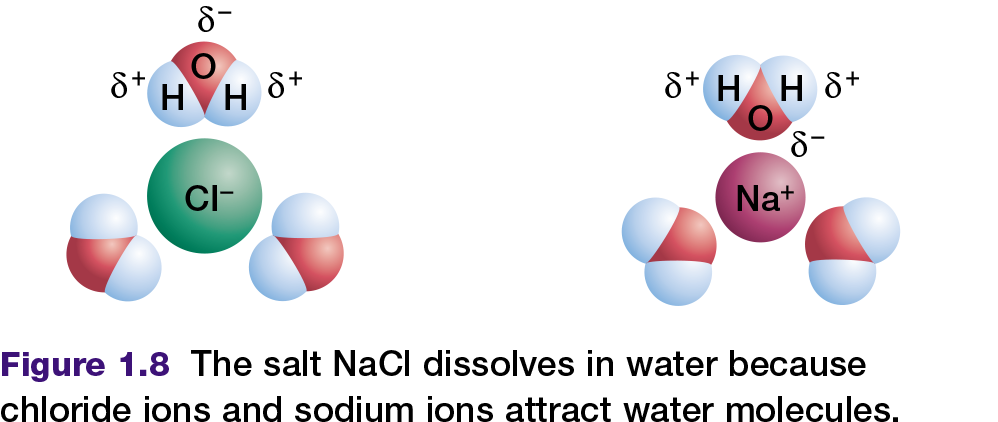
Compounds that interact with water — for example, by dissolving in it — are called hydrophilic. In contrast, compounds that do not interact with water are called hydrophobic. Nonpolar compounds are hydrophobic. They cannot form hydrogen bonds with water in the same way that ionic or polar compounds can. Therefore, hydrophobic molecules are insoluble in water. For example, when you place a drop of oil (a non-polar compound of carbon and hydrogen) into water, the oil does not mix with the water — they remain separate.
In this section, you have learned that the type of chemical bond that joins individual atoms together determines whether the resulting compound is ionic or covalent. Covalent molecules may be polar or non-polar, depending on the electronegativities of the bonded atoms and shape of the molecule.
You have learned that hydrogen bonds form between molecules in water, which interacts very differently with hydrophobic and hydrophilic compounds. Hydrophobic interactions especially have a great effect on many biological molecules. For instance, many protein molecules have hydrophobic regions in portions of their structure.
Interactions of these regions with water cause the molecules to adopt specific shapes. You will see examples of this in the next section, which reviews the four main kinds of molecules that make up all cells.
TRANSPORT IN PLANTS AND ANIMALS
Introduction
The movement of materials within the body tissues or between tissues in multicellular organisms like high plants and animals occurs in transport systems. In unicellular or low plants and animals, such movement is by the transport mechanisms of molecules within a transport medium by diffusion, osmosis, active transport and mass flow, among others.
In multicellular animals, the medium of transport is blood, while in plants, it is water in which the materials are dissolved and transported. In case of diseases such as diarrhoea, dysentery, malaria, etc, the animal body may
undergo dehydration. This causes serious effects on the body’s physiological process, which can lead to death. Just like dehydration in animals, in plants wilting or excess water loss results, especially during dry seasons or inadequate water supply or absorption by conducting tissues.
Animals and plants have specialised cells or tissues performing special functions of the transport systems apart from transporting materials.
Discuss the role of protection and support of the body structure, by the transport systems in animals and plants.
The major significance of these systems to the body has been greatly applied to economic development, for example by animal health service providers, in the construction industry using plant materials and in environmental
protection.
Background information
Transport involves the movement of materials from one part of the organism to another. In animals, it is basically the circulatory system. The system consists of the heart and blood vessels. Blood flow through the system is maintained by the heart, skeletal muscles, valves, and inspiratory movements. The heart is myogenic. Blood transports dissolved substances such as respiratory gases, water, food materials, etc.
In case of diseases such as malaria, diarrhoea and others, the body may lose blood together with water, leading to dehydration. This causes serious effects including dizziness and even death. This condition can be corrected
by administering dalose.
Explanatory note
Oral rehydration mixtures are used to boost the amount of water lost from the
body due to dehydration. Dehydration is a condition developed in patients
that have experienced illness, leading to low water amounts in blood fluid.
Rehydration helps to add water to the body so that transport of materials in
the body is efficient.
VITAL FUNCTIONS IN ANIMALS
ANIMAL NUTRITION
Nutrition in animals is heterotrophic. This means that animals feed on other living things.
Animals can be classified according to their nutrition function: herbivores (plant eaters), carnivores (eat other animals), omnivores (eat animals and plants) and saprophytic (feed on decaying organic matter).
In order to perform the vital function of nutrition, animals have different systems: digestive, respiratory, circulatory and excretory.
Digestive system
The digestive system obtains nutrients from food though the process of digestion.
In most animals, digestion takes place in the digestive tract. The digestive tract begins in the mouth, where food is torn and crushed before it is swallowed.
Next, the resulting paste goes through the oesophagus, stomach and intestines. This is where digestion is completed, and nutrients pass into the blood.
The digestive tract ends in the anus. It is through this organ that unabsorbed food is ejected though the process of defecation.
Throughout the digestive tract, there are organs called glands. They produce substances that help break down food into simpler molecules.
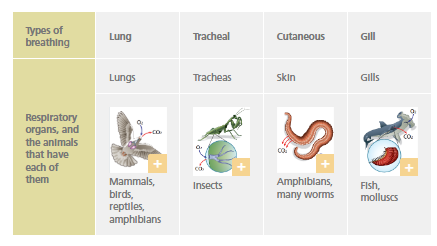
Respiratory system
The respiratory system obtains oxygen (O2) from the environment and releases carbon dioxide (CO2) that has accumulated in the cells.
Almost all animals have specialised organs dedicated to breathing, except for a few very simple animals.
Circulatory system
The circulatory system takes nutrients absorbed during digestion to the cells, as well as the oxygen absorbed during respiration. In addition, it takes and transports waste products.
This system is composed of a liquid that travels around the organism transporting substances, vessels in which the liquid moves, and one or more hearts that pump the liquid.
There are two types of circulatory system:
- Open circulatory system: in some areas, the liquid leaves the vessels and bathes the cells directly. This type of system can be found in some invertebrates.
- Closed circulatory system: the liquid always travels inside the vessels. This type of system can be found in all vertebrate animals and in some invertebrate animals.
Excretory system
Chemical reactions in organisms produce metabolic waste substances. CO2 is expelled through the respiratory system. Nitrogen compounds that could be harmful to the organism are also released. The circulatory system collects these substances and transports them to the excretory system, which eliminates them.
Nitrogen compounds are released by specialised excretory organs: nephridiums in annelids and molluscs, Malpighian tubes in insects, and kidneys in vertebrates.
ANIMAL INTERACTION
The nervous and endocrine systems perform the interaction function. The locomotive system is also involved if the response involves movement.
The nervous and endocrine systems work together to coordinate responses to stimuli.
NERVOUS SYSTEM
Animals detect changes in the external and internal environment. Most animals have sense organs to perceive external stimuli.
The information which is perceived is converted into nerve impulses. These are transmitted by the sensory nerves to the nerve centres.
Invertebrates have nodes that function as nerve centres. In vertebrates, the nerve centres are the brain and the spinal cord.
- The brain processes the information received and prepares complex responses.
- The spinal cord produces simple responses. These are called reflex actions. It also takes the information from the sensory nerves to the brain and then, from the brain to the motor nerves.
Motor nerves take the response to the effector organs that act on it. The nervous and endocrine systems are in charge of performing the interaction function. If the response involves movement, these could be muscles, or if a secretion is involved, these could be glands.
Endocrine system
The endocrine system is made up of glands in the body which release hormones into the blood in response to certain stimuli.
Hormones act specifically on certain cells, causing them to respond.
The endocrine system is involved in processes such as metamorphosis, growth and reproductive cycles. Endocrine responses are slower than nervous responses, but they last longer.
ANIMAL REPRODUCTION
Like other living things, animals carry out different types of reproduction.
- Asexual reproduction is found in some invertebrate species in two different ways:
• Budding: a new individual will develop from a bud or bulge in the body.
• Excision and fragmentation: the animal’s body divides into two parts (excision) or into several parts (fragmentation) to create a new individual.
- Sexual reproduction is the most common type of reproduction. It takes place in the reproductive system where the sexual cells or gametes can be found:
• The testicles are the male sex organs and produce sperm.
• The ovaries are the female sex organs and form the eggs.
Some species, such as snails, are hermaphrodites. Hermaphrodite organisms have both male and female sex organs, although they usually mate with another individual to reproduce.
Gametes are joined in the process of fertilisation. This process happens either inside the female’s body (internal fertilization) or outside it (external fertilization).
After fertilisation is complete, a zygote is created which divides itself to form an embryo.
Embryonic development in animals can be: oviparous, when the embryo develops inside an egg, viviparous, if it develops inside the mother, or ovoviviparous, when it develops inside an egg that remains inside the mother’s body.
- Some invertebrates, such as certain jellyfish, display alternating reproduction.
VITAL FUNCTIONS IN PLANTS
Like all living things, plants can perform the three vital functions of nutrition, interaction and reproduction.
PLANT NUTRITION
Plants use autotrophic nutrition. In order to carry out this process, they start by taking in inorganic matter through their roots and leaves:
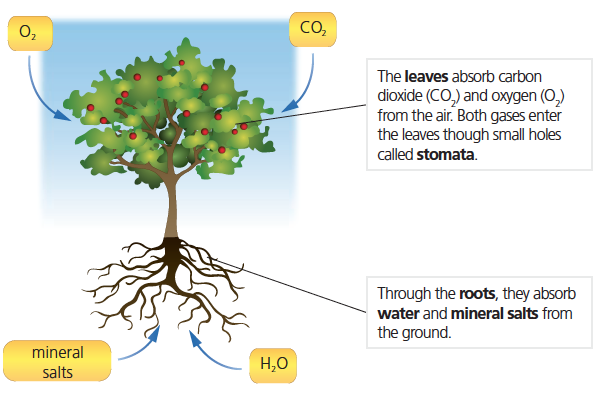
Water and the mineral salts form the raw sap which travels to the leaves through the vessels that form the xylem.
Through the process of photosynthesis, plants create organic matter from inorganic matter. This process is possible thanks to a substance called chlorophyll. It is located in the chloroplasts of plant cells, particularly in the leaves.
Chlorophyll captures energy from sunlight, and the plant uses this energy to create organic material from water, mineral salts and carbon dioxide (CO2). In this process, the plant produces oxygen (O2) and releases it into the environment.
Photosynthesis:
water + mineral salts + CO2 + sunlight g organic matter + O2
Organic matter produced during photosynthesis is shared across the whole plant as elaborated sap. This fluid travels through the vessels that make up the phloem.
The plant uses organic matter to create new structures and to perform cell respiration. This process takes place in the mitochondria. It consists of using organic matter to produce energy. During cell respiration, plants take in O2 and they release CO2.
Cell respiration:
organic matter + O2 g water + CO2 + energy
PLANT INTERACTION
Plants interact by reacting to stimuli such as light, temperature and humidity. Stimuli are also related to the growth and flowering of the plant.
Plants react to stimuli by using movement and generating hormones.
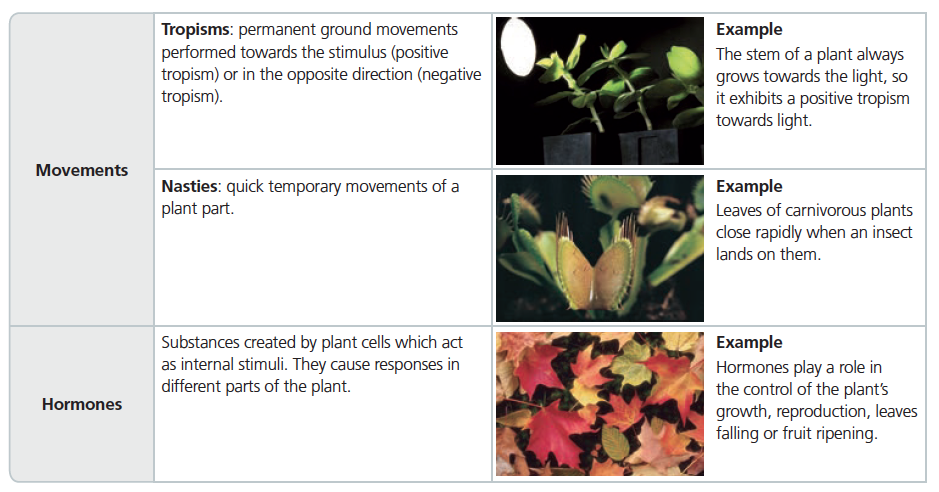
PLANT REPRODUCTION
Plants perform different types of reproduction, such as sexual, asexual or alternating.
- Asexual reproduction in plants can be exhibited in different ways:
- Sexual reproduction is characteristic of plants that produce seeds. Located in the flower are the male reproductive organs, the stamens, as well as the female reproductive organ, the carpel. The male sexual cells (pollen) are produced in the stamens, and the female ones (ovules) are produced in the carpel.
- Alternating reproduction takes place in mosses and ferns. These plants take on two different forms over the course of their life cycles: gametophyte and sporophyte. The gametophyte reproduces itself sexually by means of gametes, and the sporophyte reproduces itself asexually by means of spores.
Tasks
VITAL FUNCTIONS
In order to live, organisms use the function of nutrition to obtain the matter and energy they need.
They interact with their environment thanks to the function of interaction, and they continue to have descendants due to the function of reproduction.
NUTRITION
The purpose of nutrition is to renew and help preserve the organism’s structure as well as obtaining energy to perform the three vital functions.
Cellular metabolism is a combination of chemical reactions. This enables cells to obtain the necessary nutrients and energy to keep them alive.
In order to feed themselves, living things take substances from the environment which surrounds them, and cellular metabolism takes place in their cells.
Metabolism consists of two stages: anabolism and catabolism.
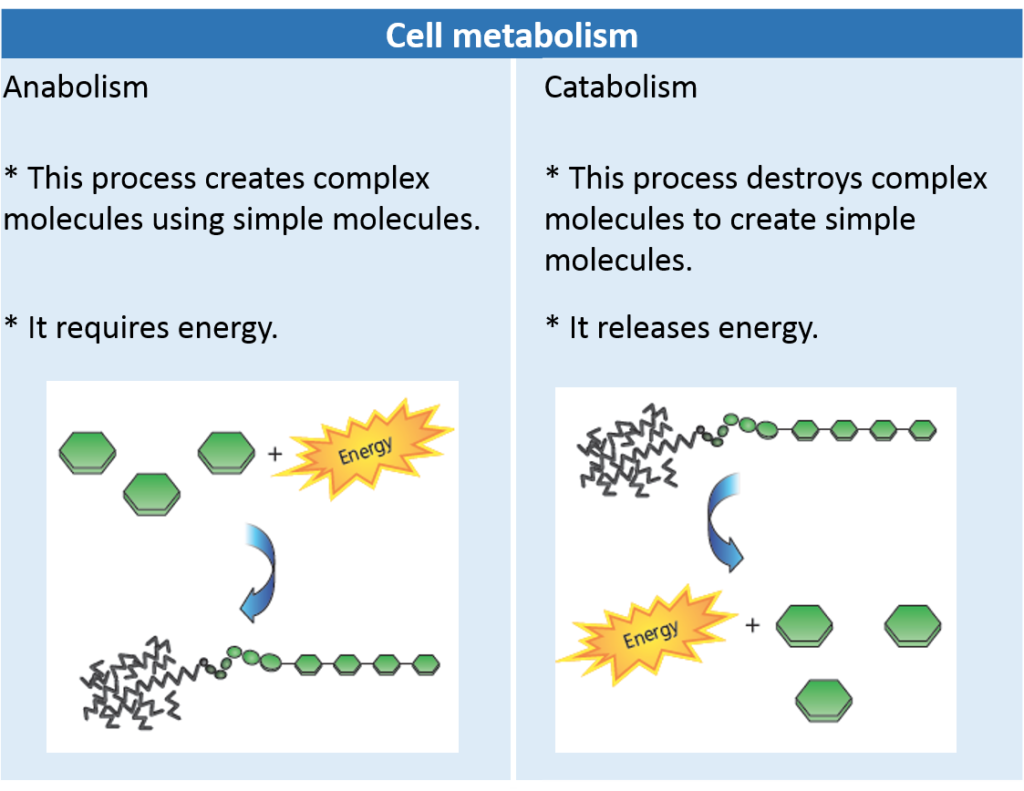
During cell metabolism, in addition to matter and energy, waste substances are released from the organism.
Nutrition types
There are two types of nutrition, depending on the type of matter obtained:
- Autotrophic nutrition: This type of nutrition is performed by organisms that obtain inorganic materials from the environment, which they transform into organic material. Plants, algae, and some bacteria perform autotrophic nutrition.
- Heterotrophic nutrition: This is performed by organisms that take organic matter from the environment. Examples of living things that use heterotrophic nutrition are: animals, fungi, protozoa and some bacteria.
INTERACTION
Interaction allows living things to interact with their environment, detect any changes in it and react to them.
Stimuli are the changes in the environment that may provoke a response in a living thing. They are perceived by the receptors, which are cells or structures specialised in perceiving stimuli.
Living things can respond to stimuli either with movements or by producing substances called hormones, which produce changes in how something functions or behaves.
REPRODUCTION
The purpose of reproduction is to create descendants and, in this way, continue the species.
There are two types of reproduction: asexual and sexual.
- Asexual reproduction: a single individual creates multiple descendants which are identical. It occurs in all unicellular organisms and in some multicellular ones. Unicellular living things only have one cell, so they can only reproduce by using the same cell.
There are three main types:
- • Bipartition: the cell divides into two cells of similar size.
- • Budding: the cell also divides into two cells, but this time they are very different in size.
- • Sporulation: one single individual divides its nucleus, creating many new individuals.
- • Sexual reproduction:it requires two individuals of opposite sex. The number of descendants is smaller than in asexual reproduction and the offspring are not identical. Multicellular organisms perform this type of reproduction.
Some species are capable of performing both types of reproduction.
Comentarios recientes